|
are generated during normal fetal development |
and Mel Greaves*§ 8242-8247 I PNAS I June 11,2002 I vol.99 I no.12 |
|
*Leukaemia Research Fund Centre for Cell and Molecular Biology,
Institute of Cancer Research, Chester Beatty Laboratories, London
SW3 6JB, United Kingdom; t Division of Transplantation Sciences,
Southmead Health Services, University of Bristol, Bristol BS10 SNB,
United Kingdom; Communicated by Janet D. Rowley, University of Chicago Medical
Center, Chicago, IL, April11, 2002 (received for review January
24,2002) Studies on monozygotic twins with concordant leukemia and retrospective
scrutiny of neonatal blood spots of patients with leukemia indicate
that chromosomal translocations characteristic of pediatric leukemia
often arise prenatally, probably as initiating events. The modest
concordance rate for leukemia in identical twins (=5%), protracted
latency, and transgenic modeling all suggest that additional postnatal
exposure and/or genetic events are required for clinically overt
leukemia development. This notion leads to the prediction that chromosome
translocations, functional fusion genes, and preleukemic clones
should be present in the blood of healthy newborns at a rate that
is significantly greater than the cumulative risk of the corresponding
leukemia. Using parallel reverse transcriptase-PCR and real-time
PCR (Taqman) screening, we find that the common leukemia fusion
genes, TELAML1 or AML1-ETO, are present in cord bloods at a frequency
that is 100-fold greater than the risk of the corresponding leukemia.
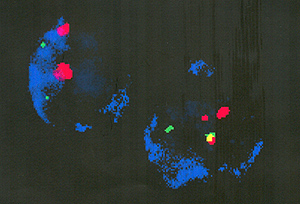
Single-cell analysis by cell enrichment and immunophenotype/ fluorescence
in situ hybridization multicolor staining confirmed the presence
of translocations in restricted cell types corresponding to the
B lymphoid or myeloid lineage of the leukemias that normally harbor
these fusion genes. The frequency of positive cells (10 high-4 to
10 high-3) indicates substantial clonal expansion of a progenitor
population. These data have significant implications for the pathogenesis,
natural history, and etiology of childhood leukemia.
|