Human Retroviruses: Linkage to Leukemia and AIDS |
|
Laboratory of Tumor Cell Biology, Na tional Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. Introduction and Background This review will discuss interactions of retroviruses with the cells of the hematopoietic system. Such interactions have been studied in the past as tools to insert genes in the cells to study their regulation or to study cellular and molecular basis of transformation in vitro. The emphasis of this review will be on viruses which cause diseases, particularly in man. A decade ago there was no general acceptance of the concept that genes were critical to leukemias and lymphomas or to disorders of hematopoietic cells. In a somewhat analogous way there was also a general feeling that viruses did not cause human cancers, and that retroviruses, in particular, did not exist in human beings. We now know that viruses, either directly or indirectly, either as a cofactor or as a direct cause, playa role in more than 40 % of human cancers. We have also learned that human retroviruses do exist and in multiple types. During the 1970s, there was also a feeling in the United States that serious or fatal, epidemic or pandemic diseases were things of the past. Infectious diseases that would become global epidemics were no longer a problem for the so-called "industrialized nations." Such diseases were really a problem for the less-privileged nations. We had preventive and curative measures like vaccines and antibiotics in addition to better sanitary conditions and public health measures. In retrospect, we should have remembered that the last great pandemic that affected the United States, Europe and the world was only about 70 years ago. It was the great influenza epidemic of 1918 -1920. And if one reviews the history of microbiology, there were often periods where epidemics disappeared and mysteriously reappeared after more than 60 or 70 years, or even for 100 or 200 years. Perhaps we were overconfident in thinking that epidemics belonged to the past: an epidemic or pandemic of the acquired immunodeficiency syndrome (AIDS) as now been with us for a decade. There was also a feeling that pandemic diseases were not possible unless the causative agents or the microbes were casually transmissible. We now know that we have a pandemic of AIDS and the agent is not casually transmissible, but transmissible only by close contact and with the exchange of body fluids. The failure to appreciate the coming of these events was probably because of the failure to remember some of the lessons of past medical history; that often there are major changes in diseases following some major changes in the society. The major changes in the post- World War II era were: a great increase in air travel; the use of blood and blood products, often going from one nation to another; the insane habit of intravenous drug abuse; and the increase in sexual contacts. All these things made it possible to transmit something that was remote or rare so that it become relatively common and global. Comparing the epidemics of the past, the AIDS epidemic is not particularly novel, nor is the response to the epidemic by the public as is often portrayed by the media. The only novel feature of this epidemic is the nature of the microbes that are causing the epidemic. The novel properties of these microbes are: they are newly discovered (but are not new); they are microbes that are often difficult to find because they do not replicate much or do not infect many target cells; they are only produced by the infected cells, primarily during the proliferating phase of the cell's life cycle. In fact, this is true for all human retroviruses. For the same reasons that they are difficult to find, the viruses are difficult to transmit. Almost always, they have a very long latency period. This is an important characteristic and has allowed their transmission to become global as they are present in the host from the time of infection until death (lifelong infection) and during that period can be transmitted to others. This is a major difference between retroviruses and other viruses, which we tend to think are transmitted while a person is sick or in the early phase of incubation, which can be a few days, a few weeks, or at the most a few months. The very long latency period of retroviruses means that it may be several years or several decades from the time of infection before the first manifestation of disease will be noted. They often cause serious diseases, e.g., central nervous system disease, malignancies, and immune deficiency. These agents have thus become increasingly important because of the serious and often fatal consequence of their infection.
There are four human retroviruses well characterized by now [1-3]. Human TIymphotropic (leukemia) virus type I (HTL V -I) was found by Gallo and coworkers in the late 1970s and first reported in 1980. Its relative, HTLV-II, was also found in our laboratory a year or two later. The human immunodeficiency virus type 1 (HIV -1) or AIDS virus causing the epidemic we now face was found in 1983 by Barre-Sinoussi et al. at the Institute Pasteur [4] and established by our laboratory as the cause of AIDS in early 1984 with many isolations of the virus and the development of the blood test. The related virus from West Africa, called HIV -2, is neither as pathogenic as HIV-1 nor is it spreading like HIV-1. It appears to be almost limited to West Africa. The technology developed in the 1970s, particularly, the sensitive assays for reverse transcriptase (R T), was crucial for the discovery of human retroviruses. The discovery of R T, by Temin [5] and independently by Baltimore [6], was quickly extended by the finding of a similar enzyme in human leukemic cells from unusual cases by Gallo and his colleagues during the 1970s [7]. Enzymes from at least four or five patients which had the properties of the viral enzyme were partially purified. More important was the development of synthetic template primers, e.g., synthetic polymers (oligo-dT -poly-A and oligo-dG-poly-C) that made the assays for these enzymes specific and sensitive. This improved the detection of retroviruses several-fold compared to the electron microscopic method used for decades. Also, the assay using RT is much simpler and cheaper and can be done continuously while the culture is ongoing. Electron microscopy does not offer that possibility. Retroviruses, including those affecting humans, complete their replication cycle much more efficiently during the proliferating phase of that cell's life cycle. R T assays performed continually on the cells in culture can reveal short-term viral replication which otherwise may be missed by electron microscopic techniques. The second important technology was the ability to grow human T cells, particularly with interleukin-2 (IL-2), discovered by Morgan, Ruscetti and Gallo in 1976 [8]. Developments in the field of immunology such as monoclonal antibodies have allowed defining subsets of lymphocytes by surface markers or by other assays to understand different functions of T -cell subtypes. A third factor contributing to the discovery of human retroviruses is the fact that they spread globally in the 1960s and the 1970s, and became much more common. We believe that this may have increased the chances of detecting and isolating them considerably. And the last point which is worth mentioning was the perseverance in looking for them, even though most scientists did not think they existed.
Human retroviruses belong to two entirely different subclasses which differ in their morphology, some aspects of their genomic organization, and some aspects of their biology. The HTLVs belong to the more classic type of animal retroviruses known in most species as type Cor oncorna retroviruses, whereas the HIVs belong to the category known as lentiretroviruses. "Lenti" is not an accurate term, as it means slow. HIV does not replicate slowly compared to HTL Vs. HTL Vs are much more slowly replicating viruses, and thus the class names can be misnomers. Until the discovery of HIVs, lenti-retroviruses were only known to occur in ungulates, the hoofed animals like horses, sheep, cows, and goats [9]. One has to be careful in not drawing too much of an analogy between HIV and these ungulate lenti-retroviruses. However, there are some common characteristics, e.g., they infect cells of the macrophage lineage and morphologically their core structures appear similar. But there are major differences in other aspects. F or example, some of the ungulate lentiretroviruses can be transmitted casually. The visna virus of sheep is thought to be transmitted by fomites in crowded sheep that are herded together in a closed environment. None of the ungulate lentiretroviruses target CD4 + T lymphocytes and they are not known to be associated with the increased frequency of the devel opment of a malignancy. More recently, we have other animal models, particularly the simian models, in which lentiretroviruses have been isolated that are closer to the humans [10]. Morphology. Biological Properties. 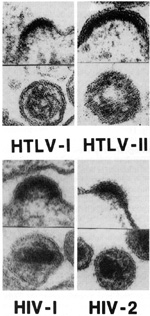
In addition, HIV destroys the cells of the immune system which are crucial in the immune surveillance itself, thereby escaping the immune attack. When the T cells are immune-stimulated, perhaps by another infection, the viral genes become active along with a variety of other cellular genes and viral proteins are expressed on the cell surface. This allows the immune system to see the infected cell. Such immune clearance may be too late. The virus released from such cells infects other cells. In this manner, the HIV -infected host who has other chronic infections is more likely to spread this virus. Modes of Transmission. Analogous Animal Retroviruses.
HTLV -I transmission is extremely tightly controlled and if one did not have a handle on the virus (virus isolation or virus detection using probes ), the diseases it causes, e.g., leukemia or neurological diseases, could be mistakenly thought to be genetically inherited. HTL V -I is endemic in Subsaharan Africa. It is not present in all parts of Subsaharan Africa, but seems to be restricted to certain tribes or geographical areas and is not casually transmitted. HTL V -I is also endemic in the Caribbean basin, including the northern part of South America, Central America, most of the Caribbean islands, and parts of the southeastern United States. Some Caribbean islands do not have any HTL V-I. It depends on where in Africa the island inhabitants have their origin. If the ancestral tribe is positive, then the descendants in some Caribbean islands are positive. Similarly, if the ancestral tribe is negative, then the descendants in another Caribbean island are negative for the most part. HTL V-l is also endemic in the southern islands of Japan in Shikoku, Kyushu, Okinawa, and other neighboring islands. Seroepidemiologic studies have suggested that clusters of HTL V -I, or a virus like it, are observed in some villages in Spain and in southeastern Italy in a region called Apulia. Manzari of Rome and Varnier of Genoa believe that the virus in Apulia in southeast Italy is endemic. It is not a coastal introduction from outside in recent times; rather, it is found in the people living in the interior hills. Recent molecular analysis studies of some of the isolates from that region indicate that it is not the classic HTLV-I, but may be another retrovirus related to HTLV-I. There have been clusters of HTLV-Irelated leukemia reported in Amsterdam and London in migrating populations from the Caribbean. The rate of developing leukemia after HTL V -I infection is identical in populations which have migrated and in the nonmigrating population, indicating that no other environmental factor is needed for the cause of leukemia, at least as far as the epidemiology can determine [11]. For a great part of the world, we have very little data. For example, we do not know very much about infection by HTL V -I in the Soviet Union.
The picture of the first patient from whom a retrovirus was isolated is given in Fig. 2. This patient was a young black male and came from the southeastern part of the United States. He had no interesting past history, either medical, familial, or occupational. He developed a severe acute T -cell malignancy of the CD4 + T lymphocytes. The skin manifestation in this disease is due to infiltrates of leukemic cells in the skin, which is a common feature in this disease [15, 16]. Frequently, there is high blood calcium, which can lead to death of the individual. Liberation of some lymphokines is suggested as a possible molecular mechanism for high blood calcium [17]. There is also an increased incidence of opportunistic infections and slight immune impairment can be observed in infected people. However, when a disease begins to develop, the course is very rapid. It resembles the chronic myelogenous leukemia going into blast crisis. Death usually follows in less than 6 months. These manifestations of the disease are common, occurring in about 70 % 80% of people who have leukemia with HTLV-1. Another 20%-30% show a more chronic course, and the diagnosis is of chronic lymphocytic leukemia of a CD 4 + T -cell type, mixed cell lymphoma, or histiocytic lymphoma of a CD4 + T cell type. So, in any CD4 + T -cell malignancy, one has to consider the possibility of HTL V -I, and particularly if the disease is as aggressive as described above. Neurologic Disease. 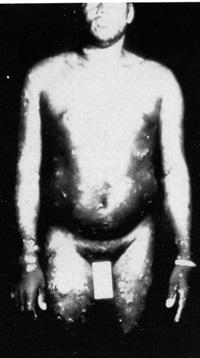
laboratories have reported HTLV –l or a closely related virus as
being involved in multiple sclerosis itself. The data are not consistent
from laboratory to laboratory. More evidence is required to implicate
HTLV-I or a relative of HTLV-I in playing a definitive role in multiple
sclerosis. However, the neurologic disease that has been called
tropical spastic paraparesis or Jamaican neuropathy, and sometimes
misdiagnosed as multiple sclerosis or a variant of multiple sclerosis,
is certainly linked to HTLV-I [18], although the disease mechanism
is not understood. The HTLV-I-associated disease differs from multiple
sclerosis in that it does not have exacerbations and remissions
like multiple sclerosis: it is progressive. It is characterized
by incontinence of the bladder, impotency in males, loss of bowel
function and spasticity of the lower extremities. The disease can
occur rapidly after infection with HTLV-I. It appears to depend
on the dose of the virus. There is a recent report of a Frenchman
who received a transfusion with HTL V -1positive blood, developed
the neurologic disease in 5 weeks, and transmitted the virus to
his wife during that period. This implies that all of the blood
supply should be tested for HTLV-I as well as for HIV [19]. However,
the neurologic disease could take many years to develop and there
is some indication that genetic factors are important. There are
some reports from Japan showing an HLA class 2 association and that
certain patterns have an increased frequency of developing the neurologic
disease. A known fact is that the virus is not found in the central
nervous system tissues, e.g., brain cells or cells of the spinal
cord, but only in the cerebrospinal fluid. The other known facts
are that people who develop the neurologic disease have a very high
titer of antibody, much higher than the healthy carriers or the
leukemic patients. Even more interesting are the recent results
of Jacobson, McFarlin, and their coworkers, who describe high levels
of cytotoxic T lymphocytes reactive Table 1. Diseases caused by or associated with
human retroviruses against tax and env gene products [20]. This has led to the speculation
that the immune response to the virus produces an autoimmune disease.
Recent reports indicate that HTLV-I is also involved or linked epidemiologically
to other diseases listed in Table 1. 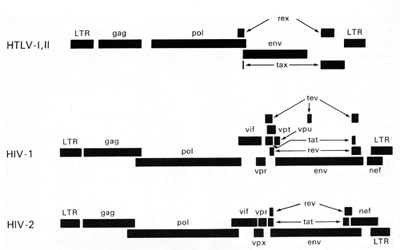
nucleus or the infected cells. The rex is the second gene in the
3' region of HTLV-I and HTLV-II. These genes are coded from two
segments of the genome and are products of doubly spliced messenger
RNAs. This phenomenon (double or even triple splicing) was new in
human retrovirology. It was soon realized that the protein products
of these genes are absolutely essential for the replication of HTL
V -I and HTL V -II. They are also essential for the biological activity
of these viruses. The products of the gag, pol, and env genes are
formed from unspliced or singly spliced messenger RNA molecules.
This is similar to what was known among animal retroviruses. 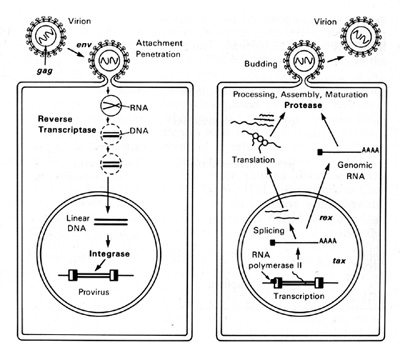
HTLV-I and HTLV-II have introduced a new complexity into our understanding of the replication cycle. and that complexity relates to the events which take place in the nucleus. In order to have successful transcription of the DNA provirus to viral RNA, first there is the expression of an early gene product. This phenomenon, although known in some DNA viruses, was newly discovered in retroviruses. The first genes to be expressed are tax and rex (Table 2). What turns on the expression of tax and rex is unknown, but the tax gene product (T AX) is essential for the early transcriptional events to make the viral RNA. The function of the rex gene product (REX) is not only newly observed in retrovirology, but it has introduced some new mechanisms into all of molecular biology. The REX protein is involved in removal or transport of the messenger RNAs for the viral structural proteins, i.e., the messenger RNAs that are unspliced or singly spliced. In other words, in the absence of REX, the only messenger RNAs that are made are the messenger RNAs that are doubly spliced, i.e., the messenger RNAs for rex and tax. But once the REX protein is made, the formation of the unspliced RNAs or the singly spliced RNAs for the viral structural proteins is favored. This is an interesting mechanism because once the REX protein is made, it down-regulates its own expression. It also down-regulates tax and allows the formation of the viral proteins so that there is a sudden release of virus during this narrow window in which these human retroviruses have to complete their cycle. This mechanism is evident even in HIV but not in the lenti-retroviruses of animals. This may suggest a convergent evolution of mechanisms for infection of human T cells by two entirely different classes of human retroviruses. Mechanism of Leukemogenesis. Table 2. Accessory genes of human retroviruses
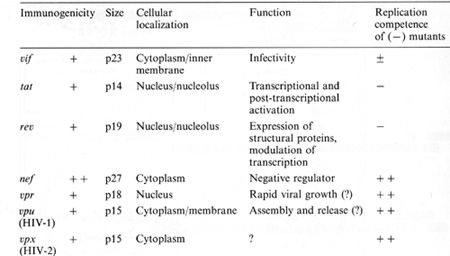 genes indirectly. It complexes to some cellular proteins and transcriptional factors that are involved in the turning on of genes important for T -cell proliferation such as those for IL-2 and IL-2 receptor (IL-2R) [22, 23]. It is somewhat ironic that the protein (IL-2) used to grow T cells to isolate the virus is the very protein that the virus uses or turns on in its first stages of leukemia. At least this is the way we think about it today. The T AX protein is also involved in turning on other cellular genes, e.g., the c-fos protooncogene. The development of adult T cell leukemia (A TL ) by HTL V -I is summarized in Fig. 5. Perhaps about one third off cells may be infected by HTL V I, but only a small fraction expresses the virus 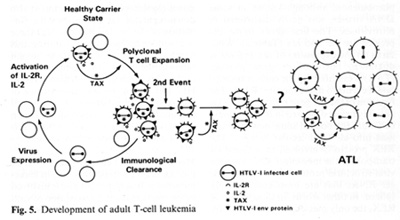
The immune system cannot see the cells which do not express the virus and cannot attack them. At some stage, the tax gene is turned on. What exactly leads to the turning on of tax is unknown, but once it occurs genes for other viral proteins can be turned on. The tax gene also turns on the IL-2 and IL-2R genes. The IL-2R has a complex structure and is made of different polypeptides. The high-affinity polypeptide of IL-2R that binds best to IL-2 is activated by tax. This may lead to autocrine and paracrine phenomena allowing polyclonal T cell expansion. At this stage it is not a malignancy but it can be documented in many people infected by HTL V -I. The immune system attacks and clears the proliferating cells expressing viral proteins. The cycles of appearance and clearance of virus-expressing cells occur for years and maybe for decades. The virus continues to increase the expansion of proliferating T cells. As estimated recently in Japan, 3%-5% of the infected individuals will be able to develop monoclonal expansion of a T cell within their lifetime, most likely mediated by another as yet unknown genetic event. This event could be an accident, a mutation, or a rearrangement, but appears to be a chance event ultimately leading to true leukemia. A third genetic event which leads to the blast crisis may be necessary, analogous to chronic myelogenous leukemia. There is no complete agreement on specific chromosomal changes to account for the second or the third event. There are some that are common, but not consistent.
The idea that AIDS might be caused by a CD4 + T -cell Iymphotropic
retrovirus came from discussions between R. Gallo and M. Essex and
his colleagues in Boston who had worked on feline leukemia virus.
Discovered in the 1960s by W. Jarrett et al. [24] in Scotland, it
was shown by W. Jarrett, 0. Jarrett, and others [25] that feline
leukemia virus can be transmitted horizontally and cause immune
deficiency as well as leukemia. Essex, in his epidemiologic studies
in the early 1980s, highlighted the greater importance of this feline
virus in immune suppression than in causing leukemia, whereas Gallo
suspected from the experiences with HTL Vs a possible involvement
of a retrovirus in AIDS. These experiences were: studies of HTL
V -I epidemiology showed that the AIDS virus was, like HTL V -I,
endemic in Central Africa; the causative agent, like the HTL Vs,
targeted CD4 + T cells; the modes of transmission by sex, blood,
and the maternal/fetal route were similar; AIDS was associated with
immunosuppression and the HL TVs can be immune suppressive (although
modestly); HTL V II had just been discovered, providing impetus
to the idea of there being more human retroviruses. All these things
led to thoughts that a new human retrovirus existed perhaps derived
from a mutation or a recombinant change in an HTLV-I emerging from
Africa, moving to Haiti and then to the United States. This was
the notion that led people, ourselves and scientists in Paris, to
look for anew retrovirus. However, ironically, we soon learned that,
though AIDS is caused by a retrovirus, the virus is not a variant
of HTLV-I or a recombinant with HTLV-I, but is due to a different
category of human retrovirus(es) that simply has (have) many properties
in common, although with a much different genomic organization as
well as classification. There are several components of the overall
pathogenesis of AIDS, the major one being the immune deficiency
with opportunistic infections. Because of the lifestyle of the individuals
there is an increased incidence of infection with real pathogens
which include mycobacteria, herpesviruses, HTL V s, and hepatitis
and papilloma viruses. In addition, there is infection of the brain
in 40% -50% of infected people. Subsequent to infection of the brain,
there is a thinking disorder and some acute psychosis. The development
of two types of tumors is very common (Kaposi's sarcoma and B-cell
lymphoma) and must be thought of as involving mechanisms distinct
from the other manifestation of AIDS. 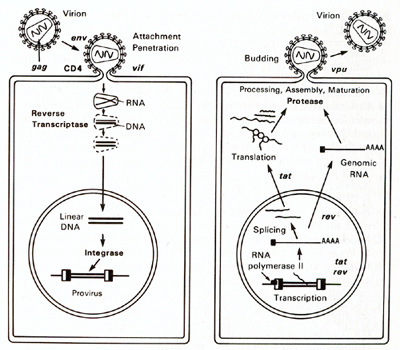
Role of HIV and HIV Proteins. The question is often asked "Why
do the CD 4 + T cells become depleted in AIDS?" HIV may be involved
in direct killing of infected T cells. HIV also has the capacity
to form multinucleated giant cells. When the virus is forming, the
envelope protein gp 120 is on the cell surface. If there are uninfected
cells nearby expressing the CD4 molecule, there will be binding
and fusion of the two cells; and this can give rise to fusion of
literally several cells together. Such cells have aberrant function
and die prematurely. Based on the laboratory observations, one can
speculate on other ways which could account for the CD 4 + T cells
depletion. Extrapolation of these to the in vivo situations may
still be remote. It is important to mention that the gp 120 falls
off the virus easily. In vitro studies show that the gp 120 can
interfere with T -cell activation. It can also lead to the down
regulation of IL-2 expression in uninfected T cells when this protein
binds to CD4 molecules. There are still other indirect mechanisms
that could permit the depletion of T cells (see Table 3).
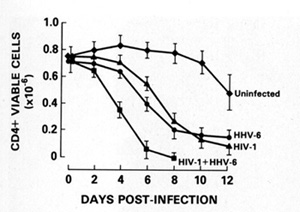
with both viruses. In addition, HIV -1infected T cells can be
activated by the simple interactions of HTL V -I with the cell membrane.
We have also shown that HTLV-I and HIV can form mixed virus particles,
which gives HIV the ability to affect cell types it normally could
not affect [36]. The new herpesvirus, human herpesvirus type 6 (HHV
-6), which we discovered and isolated in 1986 from B cells [37],
actually principally infects CD4 + T cells. This herpesvirus can
also kill T cells (Fig. 7) [38]. In the United States, 70% 80% of
all people infected with this virus are seropositive. Therefore,
in most people, it obviously causes no problem. It is the cause
of roseola in babies, which is not a very serious disease. Adults
who have antiviral antibodies and cellular immunity can control
the replication of the virus. It is possible that in AIDS with immune
impairment, there is increased replication of this virus. If so,
one must consider damage to the immune system by direct killing
of T cells by this virus. In addition, this herpesvirus can activate
the HIV genome. It has a gene which makes a protein that can trans-activate
the expression of HIV [39], analogously to the HTLV-I TAX protein.
Finally, we recently showed that this human herpesvirus is, as far
as we know, the only biological agent existing naturally that turns
on the CD4 gene at the transcriptional level, and to our knowledge
this is the first time it is known that one virus can turn on the
receptor of another [40]. In CD8 + T cells and in some epithelial
cells, infection by HHV -6 turns on CD4 so that they can become
targets for HIV infection. All these aspects of HHV -6 lead us to
propose that this virus may contribute to the impairment of the
immune system in people already immune suppressed by HIV. 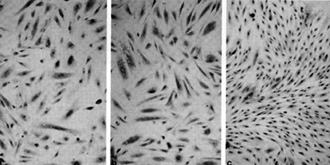 Fig. 8a-c. Spindle cells of Kaposi's sarcoma. a Culture in standard medium (RPMI 1640 plus FCS 15%). b Standard medium plus endothelial cell growth factor (ECGS) 30 µg/ml and heparin 45 µg/ml. c Standard medium plus HTL V -II CM (20% v/v) .(From [42], with permission; Copyright 1988 by the American Association for the Advancement of Science) 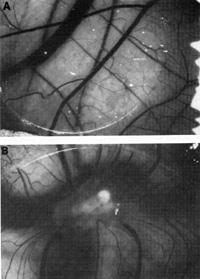
Since there has been a great increase in the incidence of Kaposi's
sarcoma in HIVinfected people, more so in male homo sexuals, one
can speculate that HIV infection plays some role. It is not known
whether any new or unknown viruses playa role in AIDS-related Kaposi's
sarcoma. We started to explore the possibility of other virus(es)
but did not find any. In the process, we developed a system for
studying Kaposi's sarcoma [41,42]. 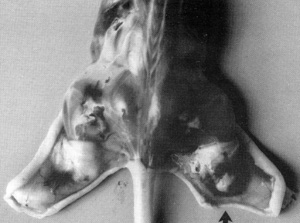
The important thing that came out of our studies over the last
few years is that we have a system in the laboratory for studying
Kaposi's sarcoma. We can grow the spindle cells which are believed
to be the tumor cells of Kaposi's sarcoma. Figure 8 shows the spindle
cells derived from a person with Kaposi's sarcoma which were grown
for several months in culture. We have several such cell cultures
now. These spindle cells have been analyzed in collaboration with
Judah Folkman and his associates from Harvard university [43]. They
have the properties of primitive smooth muscle cells of vascular
origin, as well as some properties of endothelial cells. We think
then that the precursor cell of Kaposi's sarcoma is a mesenchymal,
primitive precursor of cells of the blood vessel walls. Although
we could not find any virus, particularly HIV-1, in these cells,
it was found that they release a number of cytokines that have powerful
angiogenic activity, which is a key feature of Kaposi's sarcoma.
Figure 9 shows angiogenic activity released by the spindle cells
grown in the culture tested in the normal chick chorioallantoic
membrane [41]. One can take either the intact spindle cells or the
concentrate of factors released from them and apply it to the membrane.
Distinct angiogenic activity is observed in both instances. More
interestingly, these spindle cells, when put into a nude mouse,
cause a tumor similar to human Kaposi's sarcoma to develop (Fig.10).
The lesion develops near the site of inoculation of the spindle
cells within 10 days. When the spindle cells regress, the lesion
dies out. We examined the lesion histologically. It appears like
early Kaposi's sarcoma with blood vessel proliferation, fibroblasts,
The conclusion is that the spindle cells secrete factors that are
responsible for the early lesion of Kaposi's sarcoma [42]. We have
evaluated the cytokines it makes. It appears that IL-1 and basic
fibroblast growth factor are the most important ones. These molecules
can have angiogenic activity and promote growth of fibroblasts and
endothelial cells directly or indirectly. In addition to these,
other factors such as granulocytemacrophage colony-stimulating factor
, tumor growth factor-ß, IL-6, and low levels of acidic fibroblast
growth factor and platelet-derived growth factor are also detected
[44]. The manner in which we succeeded in growing the spindle cells
is interesting in itself. We grew the spindle cells by using Iymphokine(s)
made by chronically activated CD4 + T cells. The major active Iymphokine
for this effect is currently being purified in our laboratory and
is the most potent growth factor for AIDS Kaposi's sarcoma spindle
cells. In addition, we have found that the TAT protein is released
in very small amounts (nanograms) by HIV-1-infected T cells and
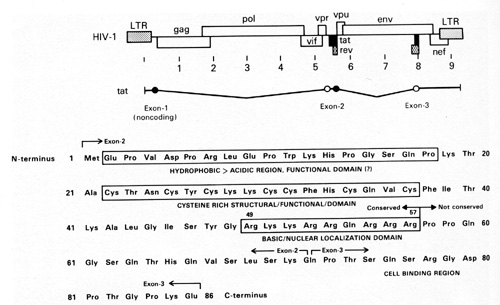
acts as a growth factor for the spindle cells [45]. The TAT molecule
has different regions which are responsible for different activities
(Fig. 11 ). We think one region is particularly important for the
growth-promoting activity on the spindle cells [45]. This small
protein of 10000 daltons is very complex. It has a region that is
important for the tran.s-activation activity of the virus and a
basic domain important for the nuclear localization. Human Retroviruses
and Tumorigenesis The direct effects of a retrovirus like HTLV-I
where the virus infects its target cell, can immortalize that cell,
and makes it abnormal have been discussed earlier. We find the viral
sequences in every cell in the sample place, indicating their clonal
derivation from the original transformed cell. HTL V -I can thus
be called a directly acting tumor virus. We refer to HIV as having
indirect effects that can lead to the increased possibility of tumor
development. HIV probably increases the possibility of Kaposi's
sarcoma developing in at least two ways: 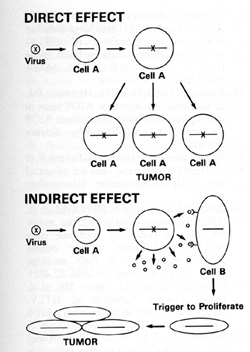
Some of these lymphokines can have an effect on the primitive mesenchymal cell that has lineage to smooth muscle and endothelium and which is the precursor of the spindle cell of Kaposi's sarcoma. This cell in turn releases a series of cytokines that act to form a complex mixed tumor that we call Kaposi's sarcoma. In summary, human retroviruses can induce tumors, directly or indirectly, in addition to their suppressive effects on the immune system and abnormal effects on the nervous system (Fig. 12).
1. Gallo RC (1986) The first human retro virus. Sci Am 255: 88
|