|
The work of the author's research group was supported
by the Justus-Liebig-Universität Giessen, by the Deutsche Forschungsgemeinschaft,
by the Bundesminister für Forschung und Technologie, and by the
Umweltbundesamt
This biennial lecture reflects the generosity of Dr. Mildred Scheel,
whose life was dedicated to the fight against cancer. I met Mildred
Scheel personally on the occasion of several conferences on human
cancer and remember her with gratitude. It is an honor to have been
invited to present the 1988 lecture. The ultimate purpose of all
who study cancer biology falls within the general goal of the efforts
of Dr. Scheel: to analyze the biological factors that are involved
in tumor development for the purpose of preventing cancer. At times
the analytical work of many scientists of Mildred Scheel's generation
appeared to meet certain opposition when they have seen printed
in large letters "cancer is not inherited" and "genes that determine
cancer do not exist." Such statements came from well-meaning people
intent on calming the fears of families that have had cancer in
their ancestry. We all arc involved in the fight against cancer,
the physicians, epidemiologists, biochemists, immunologists, virologists;
everybody in his place. I am a zoologist, trained as a geneticist
who views human beings as products of nature with all their potentials,
limitations, and inadequacies arising from their animal background.
A. Oncogenes in Phylogeny Neoplasia is not limited to human beings,
or to mammals, but develops in all taxonomic groups of recent Eumetazoa
and even in multicellular plants. Neoplasia was also found in Jurassic
Sauria and in other fossils including humans. Neoplasia, therefore,
was not created by human civilization, but is inherent in the multicellular
organization of life [1].It is, therefore, not surprising that the
genes coding for human cancer are distributed throughout the animal
kingdom (Fig. 1, [2 -10]). The most venerable oncogene seems to
be the ras oncogene, which probably has evolved together with the
heterotrophic organization of the carly Eucaryotes. This supposition
does not exclude the idea that certain sequences of ras (and other
oncogenes) might have been evolved before thc heterotrophs in the
history of life. Actually ras is distributed as a normal genomic
constituent from yeast [11 ], where one obviously cannot recognize
a cancerous state, through all groups of the animal kingdom studied
up to humans and is possibly involved in the development of human
tumors such as bladder carcinoma, melanoma, neuroblastoma, fibrosarcoma,
lung sarcoma, lung carcinoma, and acute myeloid leukemia (for review
see [12, 13]). Its early appearance in the history of life suggests
fundamental functions for our life. Its product is a GTP-binding
protein which probably activates phospholipase C that generates
the internal promoter diazylglycerol for kinase C, thus signalizing
cell proliferation [14-16].
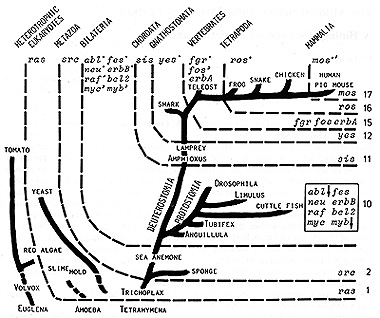
Fig. I. Attempted outline of the evolution of oncogene
systems in the animal kingdom (com piled from [2-10]). See text
As one moves up the evolutionary scale to the multicellular organization
of the living beings, i. e., to the Metazoa, the ,src oncogene appears
in the parazoic sponges and is, thereafter, traceable through the
Eumetazoa up to humans [2, 17, 18]. We have not identified cancer
in sponges, but ,src was found highly active in the sponges which,
because of the autonomy of their cells, can be considered to grow
as independently as tumors. In Coelenterata such as sea anemone
both ,src activity and abnormal growth comparable to teratomas of
higher species have been observed. High activity of src measured
as activity of its product, the pp60c-src kinase, was detected in
the nervous cell systems of all groups of animals tested. Its activity
is also high in animal and human melanoma [19, 20], the cells of
which are probably all derived from the neural crest cell-system.
The ,src oncogene is possibly, like src, involved in the transmission
of proliferation signals which, on this evolutionary level, possibly
include the phosphoinositide phosphoinositol turnover [15]. It serves
probably in intercellular communication for coordination of growth
and function of the Metazoa, perhaps through gap junctions. As we
go up to the Bilateria the Metazoa branch out to the Protostomia
and Deuterostomia, This period must have been evolutionarily very
active and successful. A large variety of taxonomic groups containing
a large packet of oncogenes has been evolved. In addition to ras
and src, the following have been identified: (a) abl, fes, neu,
erB, which belong to the src family and exhibit tyrosine kinase
function, (b) myc and myb, which are assumed to fulfill regulatory
functions of gene expression in the nucleus, (d) raf coding for
a serine/threonine kinase, and (e) bcl2, isolated from human B-cell
lymphoma. Since the viral oncogenes which mostly have been used
as probes originate from higher vertebrates (i. e., Deuterostomia),
one can conclude that the respective cellular genes must have been
already present in the last common ancestor of both Protostomia
and Deuterostomia. The clear hybridization signals always found
with abl and myb lead to the presumption that they evolved still
carlier in the history of life as can be shown by present data (see
arrows in Fig. 1). Nothing is known about the tumorigenic function
of these oncogenes in the tumors observed in invertebrates. Little
is known about these functions in human tumors [12]. abl, myb. fes.
bcl2 present in Drosophila. Limulus. etc., organisms which have
no blood in the sense of the blood of mammals, are possibly involved
in human hematopoietic malignancies; but no convincing data from
human biopsy specimens or fresh cells from a variety of human leukemias
und lymphomas are available showing that these early oncogenes are
crucial in human neoplasia [12]. The appearance of the sis oncogene,
which codes for the platelet-derived growth factor (PDG F) in the
Chordata, represented by Amphioxus and lamprey in the outline of
the phylogenetic tree, might be critical for the evolution of the
closed blood circulation apparatus that exposes the blood to pressure.
Up to the teleosts this oncogene is represented by only one copy.
Later on, moving from lower Tetrapoda to Mammalia, a second sis
copy occurs. In humans PDGF is coded by two distinct but related
genes, namely the PDGF-A gene and the PDGF-B gene, the latter one
being known as human c-sis, which is less homologous to the teleost
c-sis than the PDGF-A gene [6]. Although human c-sis is apparently
inactive in most human cells, it is supposed that both PDGF A and
B (and their receptors) are involved in general rcgulatory processes,
cell proliferation, and tumor formation [12], The yes oncogene occurs
in the animal kingdom together with the appearance of the Gnathostomata,
which are represented in our studies by sharks. This gene is a member
of the src family which is highly homologous to src itself. This
poses the question of gene duplication in evolution. Another example,
the single sis copy of the teleosts that corresponds to the human
PDGF A became duplicated (probably), as mentioned above. One could
extend this question asking whether the large src family including
the already mentioned ab/,.fes. neu. erbB, yyes and the not yet
mentioned fgr, ros, and mos could have been evolved by gene duplication.
The idea that oncogene families might have been evolved by gene
duplication contributes to the general concept of evolution by gene
duplication proposed by Ohno [21] almost 20 years ago. At the evolutionary
level of Vertebrates, fgr, a member of the src family, fos, a nember
of the myc/myb family, and erbA, a partial homolog of the receptors
of thyroid hormone, estrogen, progesterone, glucocorticoid hormone
of humans, and the human X-factor, appear together in the teleosts.
Since erb A of the fish shows strong homologies to the viral gene,
one could assume that it has evolved earlier in the history of life
than the present data indicate. It seems not to be involved in neoplastic
transformation but in tumor promotion, perhaps supporting erb B,
which appears to be involved in transformation [22]. It is notable
that, based on our earlier genetic and histogenetic experiments,
not only have gene patterns favorable to neoplasia been observed
in teleost species but also genes which limit the action of these
genes to certain cell types [23]. This is an important point to
consider in human neoplasia [3]. It appears that nature's way of
keeping the oncogenes from their transforming capacity as soon as
they became too dangerous for the increasing complexity of life
has been to establish a new category of genes, namely the oncogene-specific
regulatory genes [24], today sometimes called anti-oncogenes or
oncostatic genes. Finally, ros. a member of the .src family, possibly
involved in cell proliferation and tumor promotion through the internal
promoter diazyglycerol [14-16], appears to be specific to the Tetrapoda,
and mos, related to the src family and also to raf, appears to be
specific to Mammalia [4, 5, 25]. Nothing is known, at least to my
knowledge, about the specificity of these genes to the organization
of the Tetrapoda and Mammalia, respectively. mos is probably involved
in human acute myelogeneous leukemia [12]. In conclusion it appears
that, in parallel with the advancement of the animal kingdom, particular
oncogenes were subject to their own evolution and that, furthermore,
the systems of the oncogenes corresponding to this advancement increased
in number, several of them probably by gene duplication. From yeast
to mammals we found an increase from 1 to 17 (see Fig. 1, right).
This increase might reflect the increase of complexity required
for advancement in the anima] evolution but might in addition reflect
an increase of sensitivity to any endogenous and exogenous impairment
of the systems. Therefore, our phylogenetic view might reflect some
rough observations on the tumor incidence in the animal kingdom
which so far have never been studied seriously. Although both oncogenes
and cancer have been observed in all systematic categories of the
Eumetazoa, it appears that mammals are more afflicted with cancer
than any other group of animals.
B.Low and High Susceptibility to Neoplasia
Neoplasia occurs infrequently in the natural populations of Eumetazoa,
and induction of cancer by initiating carcinogens and tumor promoters
is difficult to achieve [26]. This phenomenon was studied in detail
in the Central American teleost genus Xlphophorus [26-29] and in
East Asiatic mice [30]. Natural selection in Mendelian populations
will not favor one population or race and discriminate against the
other but will always work against susceptibility to cancer in all
populations and races. However, certain nontaxonomically defined
groups of animals are highly susceptible to spontaneously developing,
carcinogen-initiated, and promoter-stimulated neoplasms (Table 1).
These groups consist mainly of animals of hybrid origin, such as
naturally occurring or experimentally produced interspecific, interracial,
and interpopulational hybrids as well as laboratory and domesticated
animals which actually are also hybrids, i. e., homozygous combinations
of chromosomes of different populational or racial provenance. These
animals share their high susceptibility to neoplasia with humans
[26, 31]. While we do not have data on the relationship between
hybridization and cancer in human beings comparable to the data
on animals, it is interesting to speculate whether the many facts
on tumor incidence in humans that do not agree with the concept
of the primacy of environmental factors in carcinogenesis can be
explained by interpopulational and interracia1 matings in our ancestry.
Certainly interpopulational and interracial mating may have occurred
at any time in any place. Because of the high and increasing mobility
of modern humans as compared with other species, one should expect
high heterogeneity. Various estimates based on enzyme variation
showed that heterogeneity in humans is comparable to that of domestic
animals such as cats, but is about six times higher than that of
wild macaques, about ten times higher than that observed in the
large wild mammals such as elk, moose, polar bear, and elephant
seal, and about twice as great as that of most feral rodents studied
so far [32 34]. Based on these
Table I. Animals that exhibit a high tumor
incidence (for references see [26, 31])

data and on the assumption that tumor incidence in general is related
to interpopulational and interracial matings, one could explain
why humans have a high incidence of neoplasia comparable to that
of the domestic animals. Furthermore, there are some data on chromosomal
heteromorphisms in human populations that might be useful for estimates
of heterogeneity within and among different populations. According
to such estimates it appears that, for instance, Japanese populations
exhibit a low degree of Q- and C-band chromo some heteromorphisms.
whereas Americans have a much higher degree of this heteromorphism,
with blacks having more prominent heteromorphisms than whites [35,
36]. One is tempted to assume that this heteromorphism refects the
differences in the degree of heterogeneity among the Japanese and
white and black United States populations. In this context it is
notable that the ratio of prostatic cancer in Japanese, United States
whites, and United States blacks is reported as 1: 10: 30 and that
the black citizens in San Francisco have double the risk of developing
neoplasia as compared with their Japanese fellow citizens [37, 38].
We cannot explain these facts by environmental factors or racial
differences. The high susceptibility to neoplasia in domestic or
hybrid animals, respectively, could show us how to approach the
problem. Of course, it is very difficult to study the heterogeneity
of a recent human population of a city or country in terms of biological
measures. However, new methods such as the determination of restriction
fragment length polymorphisms available today could be helpful in
revealing the possible relationship between genetic heterogeneity
and tumor incidence in modern human populations.
C. Cancer in Xiphophorus as a Model for Cancer in Humans
Human biology is unique, but is not so unique in its fundamentals
as to make studies on animal models irrelevant for an explanation
of human diseases including cancer. Although mice and rats are the
classical laboratory animals used in experimental cancer research,
several genera of small teleost fish serve increasingly as models
in new cancer research programs [39]. One of these genera is Xiphophorus
(Fig. 2; for portraits of different phenotypes see [2, 3, 22, 23,
29, 31]), the animal model from Central America that we have used
in our laboratories for 30 years [24,40]. Neoplasia appears to develop
only very exceptionally in the wild populations of xiphophorine
fish. In spite of the fact that thousands of individuals of many
wild populations that are isolated from each other have been collected
by several investigators and myself, no tumor has been detected.
In the progeny of the wild populations that have been inbred in
the laboratory for about 8o- 100 generations, no tumor has occurred
spontaneously and almost

Fig. 2. Female and male of the "spotted dorsal'
platyfish, Xiphophorus maculaIus, from Rio Jamapa (Mexico)
Table 2. Oncogenes in Xiphophorus

no tumor could be induced even with the strongest mutagens-carcinogens
such as X-rays and N-methyl-N-nitrosourea (MNU). This fact requires
special clarification since most of the oncogenes that are known
to transform the cells and to drive the tumors are present in the
fish (Table 2). If, however, interpopulational and interspecific
crossings are performed, depending on the genotype, the progeny
spontaneously or following treatment with initiating carcinogens
(Xrays, MNU, ethylnitrosourea, diethylnitrosamine, 2-amino-3-methylimidazo(4,5-f)quinoline,
etc.) and/or tumor promoters (12-0-tetradecanoylphorbol-13acetate
= TPA, 5-azacytidine, phenobarbital, cyclamate, testosterone, nortestos
terone, methyltestosterone, trenbolone, ethinylestradiol, cAMP,
biphenyl, butylhydroxy toluene, deoxycholic acid, thioacetamine,
bis(2-ethylhexyl)-phthalate, betel nut extract, etc.) develops neoplasia
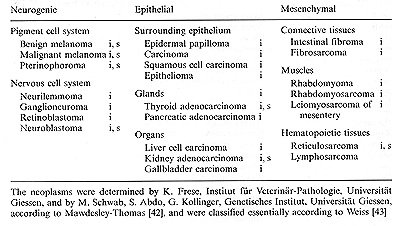
(data in [41]). Neoplasms originate from all neurogenic, epithelial,
and mesenchymal tissues (Table 3). The suitability of the model
is, except for research on mammalian-specific tumors such as breast
cancer, lung cancer, etc., beyond question and its efficiency is
more economic and time-saving than that of the laboratory mammals.
Agents that induce neoplasia in certain high-risk genotypes of the
fish hybrids, might, in principle, also affect certain high-risk
human individuals.
Table 3. Neoplasms in xiphophorine hybrid fish
induced by physical and chemical agents (i) or spontaneously developed
(s)
D. Classification of Tumor Etiology in Xiphophorus and Humans
The neoplasms of Xiphophorus can be classified 1. Mating conditioned:
accessory oncogenes are introduced into, and/or regulatory genes
for the oncogenes are eliminated from, the germ line by replacement
of chromosomes carrying the respective genes or lacking them, and
vice versa. 2. Mendelian inherited: regulatory genes for oncogenes
are impaired, lost, or dislocated in the germ line by mutation.
3. Mutagen-carcinogen conditioned: regulatory genes for oncogenes
are impaired, lost, or dislocated in a somatic cell by mutation.
4. Nutrient and endocrine conditioned: resting stem cells are pushed
to differentiate by tumor promoters (the genetic preconditions according
to a, b, and care fulfilled by earlier events). 5. Virus conditioned:
accessory oncogenes are introduced (so far not convincingly shown
in the fish). The same classification can be applied to human cancer
comprising a small group of (a) "familial"; (b) "hereditary" neoplasms
in which genetic factors are supposed to be involved, e. g., retinoblastoma,
meningioma, melanoma; (c) a large group of "carcinogen-dependent"
neoplasms, e, g., lung cancer; (d) a large group of "endocrine-dependent"
and "'digestion-related" neoplasms, e. g., breast, prostatic, colon
cancer; and, finally, (c) a group of viral-conditioned neoplasms,
e. g., leukemia, genital tumors. In Xiphophorus derived from a wild
population neoplasia develops in general only if different protocols
for the induction of tumors are combined by the experimenter, for
instance, (a) the elimination of regulatory genes by selective matings,
(b) the induction of germ line mutations, and (c) the induction
of somatic mutations, etc. The particular events that alone do not
lead to neoplasia, summate, and appear as a multistep process that
goes beyond the generations and, finally, reaches the last step
that leads to neoplasia in a certain individual. The experimenter
must detect the sequence of the different steps, and it is easy
to see that the last step that completes the multistep process determines
the etiological type of neoplasia. This was shown for Xiphophorus
but might be helpful to explain the different types of tumor etiology
in humans in which both the ancestry of an individual and the individual
itself are involved. In the following paragraphs we shall try to
approach the biological basis of spontaneously developing, carcinogenmutagen
induced, and promoter-dependent neoplasms.
E. Tumors Appearing and Disappearing in the Succeeding Generations
Human tumors such as a certain colon cancer that afflicts individuals
15-20 years sooner than generally may appear ..spontaneously" in
a family in one generation and may disappear in the succeeding generation.
This is demonstrated by means of a cartoon (Fig. 3, upper part)
adapted from Lynch and his colleagues [50]. We cannot explain this
phenomenon. The Xiphophorus model (Fig. 3, lower part) provided
the opportunity to study a similar appearance through the fish generations.
Crossings of a spotted platyfish (A) with a nonspotted swordtail
(B) result in F 1 hybrids (C) that develop enhanced spot expression
and sometimes benign melanoma instead of the spots. Backcrossings
of the F 1 hybrids with the swordtail as the recurrent parent result
in BC1 offspring (D, E, F), 50% of which exhibit neither spots nor
melanomas (F) while 25% develop benign melanoma (D) and 25% develop
malignant melanoma (E). Further backcrossings of the fish (not shown
in Fig. 3) carrying benign melanoma with the swordtail result in
a BC2 that exhibits the same segregation as the BC1. As opposed
to the crossing procedure that gave rise to the melanoma, backcrossings
of the melanoma-bearing hybrids (E), with the platyfish as the recur
rent parent (A), result in an alleviation of the melanoma in the
offspring (C*), which in the following BC generation grow into healthy
fish (A *). In conclusion, malignant melanoma of the BC animal (E)
originates from the spots of the preceding platyfish generations
(A) and is reduced to spots again in succeeding generations (A *).
The formal parallelism in the occurrence of neoplasia in the human
family and in the experimental model is striking. In our search
for causes of human cancer there might be some value in realizing
the types of factors that can be passed from the fish parents to
the fish offspring to influence the occurrence of cancer. The experiment
with the model suggests that certain human cancers may be expected
to occur in individuals because of a combination of factors from
both parents that by themselves did not cause cancer in either parent.
More data are required in order to compare more stringently human
familiar cancer with mating-conditioned neoplasia in the model.
F. Oncogene Expression in the Tumors
The appearance of tumors in both human and model brings about the
question for the oncogenes expressed in human and xiphophorine neoplasms.
Data available for melanoma indicate an elevated expression of both
the human and thc xiphophorine src, erbB, sis, ras, and myc ([2,6,7,18-20,40,44,45]
personal communication, u. Rodeck). Measurements concerning the
significance of the xiphophorine src oncogene (X-src) for the development
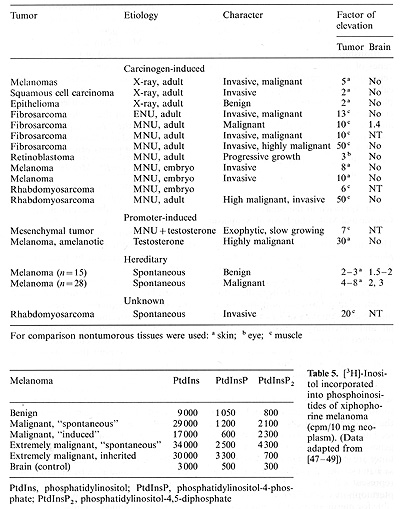
of melanoma and other kinds of neoplasia in the fish (Table 4) showed
that the activity of its product, the pp60x-src kinase, may be elevated
in the tumors up to 50 times over that of the controls [46]. Furthermore,
the phosphoinositide phosphoinositol turnover , which is supposed
to be linked to the .X-src activity [14-16], was found up to more
than ten times elevated over that of the controls (Table 5). This
finding is important because the turnover may serve as a measure
for the activation of phospholipase C, which generates the internal
promoter diacylglycerol.
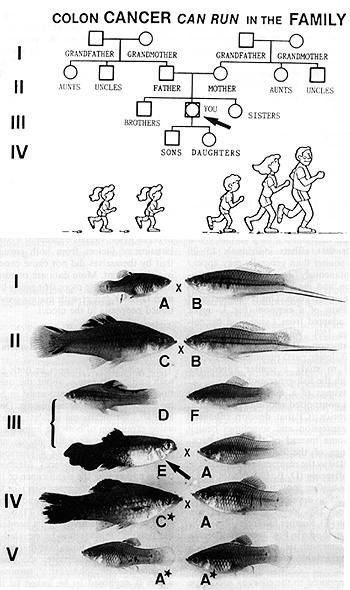
Fig.3. Appearance and disappearance of neoplasia
in succeeding generations (cartoon adapted from [50]). See text
Table 4. Elevation ofpp60x-src kinase activity in tumors
and brain of Xiphophorus hybrids. (Data from [46])
A tremendous amount of work on oncogene expression and its possible
secondary processes in the tumors and in tumor-derived cell lines
of experimental mammals and of humans [12] has been performed in
the expectation of finding a particular tumor type-specific initial
gene and the initial event of the formation of a particular neoplasm.
While we were never able to identify what one could term a "liver
cancer gene" or a "melanoma gene", others have thought they did.
Our own studies on the Xiphophorus model showed only a relationship
of a number of regulatory genes of a number of tissue-specific developmental
genes which in total we called "tumor gene-complex" (Tu complex);
but we interpreted this as an association rather than a true genetic
entity, and we assigned the different kinds of neoplasms such as
those listed in Tables 3 and 4 to the same Tu complex. The nature
of the causality of neoplasia remained unclear.
G. An Approach to the Study of the Genetic and Molecular Basis
of Neoplasia
The genes underlying neoplasia in Xiphophorus were most successfully
studied in the generations developing the "spontaneously occurring"
mating-conditioned tumors, and it appears to be in the nature of
things that those laboratories working presently on the small group
of familial and hereditary human tumors approached the fundamentals
of neoplasia at least as closely as those working on the large groups
of carcinogen- and promoter-dependent tumors. Our approach in the
model is described by means of Fig. 4, which refers to the same
fish as indicated in Fig. 3 by the same capital letters (for the
mutants see later). Based on breakpoint data the genes responsible
for melanoma inheritance are located terminally in one Giemsa band
of the X chromosome [51] and represent a complex consisting of (a)
the pterinophore locus (Ptr) which is responsible for pterinophore
differentiation, (b ) the compartment-specific dorsal fin locus
(Dr, impaired to Dl,) which restricts both pterinophore and macromelanophore
differentiation to the dorsal part of the body, ( c) the region
in which a viral erb Brelated oncogene (erb B*, an oncogene related
to the receptor of the human epidermal growth factor, EGF, x-egfr)
is located, (d) the melanophore locus (Mel), which appears to be
under control of DI and erbB*, and (e) the arbitrarily symbolized
"tumor gene" (Tu), which appears as a Mendelian factor but might
possibly be composed of both erb B* and Mel [22, 52]. Oncogenes
in addition to the xiphophorine erb B* (x-erb B*) could not be detected
in the X chromosome. Based on our present knowledge, the respective
region of the X chromosome of the platyfish, the "Tu complex," can
be roughly mapped as follows (commas represent breaking points observed):
X ….Ptr, Df, erbB*, Mel-Tu At least about 20 linked genes are involved
in the regulation of the Tu complex, but there are also several
nonlinked regulatory genes, e. g., the Diff gene, which, if present
in the homozygous state, restrains the transformed pigment cells
from proliferation by terminal differentiation [53]. The swordtail
(B) has neither evolved a comparable Tu complex nor the linked and
nonlinked regulatory genes. Since platyfish and swordtails have
a rather high number of chromosomes (11 = 48) and since clear-cut
chromosomal conditions concerning their origin were required, the
experimental animals, besides the purebreds (A, B), were taken from
the F 1 (C), which contains one platyfish and one swordtail genome,
and from high backcross generations comprising BC8 up to BC22 (F,
E), the genome of which virtually consists of swordtail chromosomes
except for the Tu complex containing X chromosome selected from
the platyfish by the crossings. The phenotypic overexpression of
the Tu complex thus depends mainly on the crossing-conditioned replacement
of platyfish autosomes carrying regulatory genes such as the differentiation
gene D iff: by swordtail autosomes lacking such genes. More information
about the Tu complex comes from studies on the restriction length
polymorphism of the oncogene
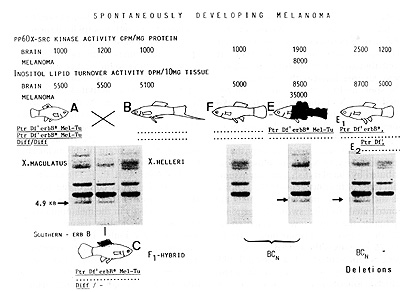
Fig.4. Appearance of mating-conditioned development of
melanoma after crossings of X. macu/alus x X. he//eri (platyfish
x swordtail; A x B) and backcrossings of the F 1 hybrid (C) with
x. he//eri. F and E represent the backcross generation (BCn). E1
and E2 represent deletions. The fish indicated by the capital letters
correspond to those indicated in Fig. 3 by the same letters. Note
that the 4. 9-k b Eco R 1 Southern fragment is inherited along with
the tumor gene-complex. Ptr, pterinophore locus; Df, impaired dorsal
fin-specific regulatory gene; erbB*, xiphophorine copy of an oncogene
related to the viral erb B; Me/-Tu, melanophore locus containing
the potential for tumor formation. Diff: a nonlinked differentiation
gene;-, chromosomes of x. maculatus; … , chromosomes or X, helleri.
See text
derived from platyfish and from swordtail. Some of the xiphophorine
oncogenes (x-oncs) listed in Table 2 show restriction fragment length
polymorphism (RFLP) the patterns of which have been differently
evolved in the wild fish of different provenance [6-8,22, 40]. For
instance, the patterns of the lengths of the restriction fragments
of x-sis are specific to each of the different species, but show
no RFLP within each of the species; actually these species show
a monomorphism of the restriction fragment lengths of x-sis. In
contrast, the patterns of the lengths of restriction fragments of
x-erbA and x-erbB are species nonspecific, but are specific to the
different races and populations of the species. The lengths of certain
fragments of x-erbB are even different in females and males of the
same population. We used the RFLP phenomenon as an indicator for
the Mendelian inheritance of the x-oncs through the purebred and
hybrid generations. If a certain oncogene fragment is independently
inherited from the inheritance of spot or melanoma formation, then
one can conclude that the respective oncogene is not "critical"
for the first step of melanoma formation. This is not to say that
such an oncogene is not involved in melanoma formation at all; as
already mentioned, x-src, x-sis, x-ras, x-myc are expressed in the
melanomas and are certainly involved in tumor growth or tumor progression,
but they are not involved in the first step leading to melanoma
because they are contributed by the swordtail to the hybrid genome
whereas the appearance of the spots and the melanomas is contributed
by the X chromosome of the platyfish. Furthermore, since 47 chromosomes
of the malignant melanoma bearing backcross hybrids are contributed
by the swordtail and only 1, name]y the Tu complex carrying X chromosome,
is contributed by the platyfish, one can assume that most of the
oncogenes in the genome of the tumorous backcross animals are contributed
by the swordtail genome. Actually, the only x-onc detected so far
on the platyfish chromosome carrying the Tu complex is the x-erb
B*. This oncogene is represented in Fig. 4 by a 4.9-kb Eco Rl Southern
restriction fragment which is inherited along with spot and/or melanoma
development (A, C, E) and is lacking in the melanoma-free swordtail
(B) and the melanoma-free BC hybrid (F). The other EcoRl fragments
that a]so indicate erb B sequences could not be assigned to the
X-chromosomal locus where the inheritance of the melanomas comes
from. Additional information about the correlation between the inheritance
of melanoma formation and the inheritance of the x-erb B*-representing
4.9-kb Southern fragment comes from two mutants of the type E BC
hybrids. Both types (Fig.4, El and El) have lost the locus Me/-Tu,
i.e., the capability to develop melanoma, but only one type (El)
has also lost x-erb B* as is shown by the ]ack of the 4.9-kb fragment.
This result indicates that (a) x-erb B* is located between Dfand
Me/-Tu and (b) information crucial for melanoma formation depends
on Me/- Tu, which codes for the differentiation of certain pigment
cells. This is, however, not to say that there are no links in the
chain of events leading to the very beginning of melanoma formation
that precede the function of Me/-Tu. As was already mentioned, pp60x-src
kinase activity and inositol lipid turnover activity was found enormously
elevated in the melanomas. This is true for all kinds of tumors
so far studied and for all types of tumor etiology (Tables 4, 5).
Unexpectedly, these activities were also found elevated in the healthy
tissues of the fish carrying mating-conditioned and Mendelian-inherited
melanomas. figure 4 (upper part) shows the rounded data measured
in the brain of the melanomatous BC hybrids type E in comparison
to those of types A, B, C, F. The results suggest that the genes
controlling pp60x-src and the inositol lipid turnover are expressed
not on]y in the melanoma tissues but also in the healthy tissues
of the tumorous individuals, independently of whether they are involved
in neoplasia or not [46, 49]. Possibly this phenomenon corresponds
to the often-occurring multiple tumors in combinations such as melanoma,
neuroblastoma, rhabdomyosarcoma, and retinoblastoma in the BC segregants,
sometimes even in a particular anima]. Multiple tumors and cancer
family syndromes have been reported also in humans [54]. The working
group of Lampert [55], for instance, studied a family which, despite
a healthy ancestry, developed neuroblastoma, ganglioneuroma, and
other neurogenic tumors running through two generations. Lynch and
his colleagues [ 56] reported the pedigree of a family afflicted
with cancer on breast, urinary b]adder, brain, colon, cervix, endometrium,
pancreas, prostate, skin, stomach, and uterus. We cannot explain
this phenomenon, but the model shows us the possibility of an approach
to the study of some of its molecular and biochemica] fundamentals.
It appears that the measurements of pp60x-src kinase activity and
inositol incorporation into phosphoinositides in the brain of the
deletion mutants of the fish which are incapable of developing melanoma
(Fig. 4, right) open new possibilities for intervention in key signals
critica] to the endogenous induction of
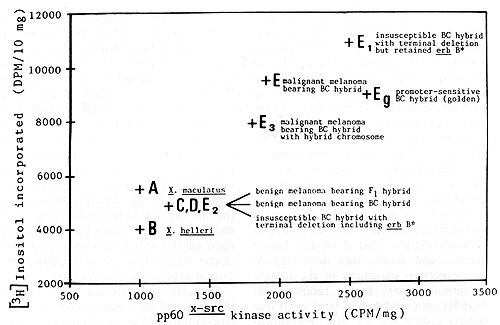
Fig.5. Incorporation of [³H] inositol in phosphatidylinositol
in brain extracts, plotted against activity of pp60x-src .Capital
letters correspond to the fish indicated by the same letters in
Figs. 3 and 4; E3 ist not shown. Eg corresponds to the promoter-sensitive
fish shown in Fig. 9 (on right) Note the high correlation of both
parameters. Data from [49]. See text
neoplastic transformation in the animal model and possibly in humans.
Both pp60x-src kinase activity and inositol lipid turnover activity
are highly elevated in the brain of those insusceptible deletion
animals that have lost the Mel- Tu locus but have retained the x-erbB*
oncogene (Fig. 4, E1). In contrast, the deletion animals having
lost the x-erb B* together with the Mel- Tu locus (E2) exhibit no
elevation. This result suggests that the molecular and biochemical
machinery supposedly involved in melanoma formation may be running
for genetic reasons, without forming melanoma. Our results, moreover,
suggest that there may be a particular type of activation of x-.src
and the inositol phospholipid system that is a marker for predisposition
to cancer and could be used for the determination of pro-neoplasia
conditions in cancer risk studies. Support for this suggestion comes
from the excellent correlation existing between pp60x-src kinase
activity and the [³H]inositol incorporation into phosphatidylinositol
(Fig. 5). One more suggestion arises if one compares the different
results obtained with the E1 and E2 BC hybrids. Because of the backcross
procedure applied to the animals most of the genes involved in melanoma
formation are contributed to the hybrids by the swordtail genome.
In the deletion hybrid E2 lacking x-erbB* they appear to rest in
low activity, indicating that, in order to become involved in melanoma
formation, they require a signal for the change from a resting to
activated state. The results obtained with the deletion hybrid El
show that this signal is transmitted from that region of the Tu
complex containing platyfish chromosome where x-erb B* is located
and where the inheritance of the melanoma is determined. In conclusion,
based on the possibility of distinguishing between genes originating
from platyfish and swordtail in the genome or certain hybrids, we
round that development and growth or melanoma is mainly run by a
set or genes that requires a signal for its activation which, due
to the onset or the crossing experiments with the mutants, is transmitted
from an x-erb B*-containing chromosome locus. This locus, however,
is probably deregulated by the crossing-conditioned replacement
of platyfish chromosomes carrying regulatory genes for the Tu complex
(i. e., probably x-erb B*) by swordtail chromosomes lacking them.
The 4.9-kb Eco R1 restriction fragment was cloned, subcloned, and
sequenced. It contains exon c and d or the kinase domain and shows
high homology to the respective sequences or the human epidermal
growth factor receptor (HEGFR) gene and to the viral erbB (for complete
data see [22, 40]). Hybridization or this xiphophorine fragment
against genomic xiphophorine DNA revealed the presence or highly
homologous sequences located on the Y-chromosome (6.7 kb; see later),
on the Z-chromosome, and on an autosome present in all individuals.
Another species, Xiphophorus variatus, which was studied for comparison,
also exhibited an homologous fragment which is inherited along with
tumor susceptibility. Each of the x-erb B* copies corresponding
to these homologous fragments from different chromosomes is also
part of a Tu complex [40]. Hybrids carrying these Tu complexes,
however, require treatment with carcinogens as a precondition for
melanoma development.
H. Carcinogen-Dependent Neoplasia
The remainder or my review or human cancer is devoted to the large
groups or mutagen-carcinogen conditioned (somatic mutation conditioned)
and nutrient and endocrine conditioned (promoter conditioned) neoplasms.
Both types or etiology comprise probably more than 90% of all tumors.
A large body or consistent and contradictory observations on their
causation are available. Lung tumors or humans probably offer the
most convincing observations on the involvement or exogeneously
induced somatic mutations in the initiation or the tumor. They appear
not to be influenced by many environmental factors, and there is
no evidence that hormonal or nutritional factors are involved in
their causation. The simple interpretation or the induction or a
somatic mutation by a physical or chemical carcinogen, however,
does not explain the different susceptibility or the different individuals
that are exposed to the carcinogen. There must exist hereditary
factors that enable most of the individuals to escape lung cancer
while others become victims. We cannot explain this observation.
Recently Newman and her colleagues [57] reported on breast cancer
in an extended family (Fig. 6). A complex segregation analysis indicated
that susceptibility to breast cancer in the family can be explained
by autosomal inheritance or a defective regulatory gene while the
appearance or the tumor requires a somatic mutation in a target
cell. This example shows that steps toward breast cancer had already
occurred unnoticed in the preceding generation; the somatic mutation
represents only the last step that completes the chain or events
leading to cancer. The Xiphophorus model provided more details for
the study or the complex situation in the somatic mutation-dependent
tumors. In mutagenesis studies [52] we detected nontumorous hybrid
genotypes which, following treatment with directly acting carcinogens
(X-rays, MNU), develop after a latent period or 8-12 months foci
or transformed pigment cells that grow out to compact melanomas
(Fig. 7). The smallest cell clones to which these melanomas could
be traced consisted or eight cells indicating that there were three
cell divisions between a somatic mutation event and the occurrence
or the transformed pigment cells [23]. The incidence or these tumors
depends on the dosage or the treatment, and may reach up to 100%.
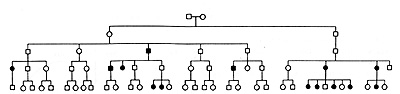
Fig. 6. Pedigree of a family at high risk of breast
cancer, adapted from(57). See text

Fig.7. Mutagen-carcinogen-sensitive fish developing MNU-induced
melanoma. Note the closely circumscribed growth reminiscent of the
somatic mutation-conditioned unicellular origin of the tumor
These observations led to the assumption that the Tu complex of
the treated hybrids is under control of only one regulatory gene
which, following treatment, is impaired in a particular pigment
cell. Assuming the total of the pigment cell precursors that are
competent for neoplastic transformation is 10 high 6 (this is the
average number in the pigment cell sys tem of young fish), and the
induced mutation rate is 10 high-6, then the tumor incidence is
1 ( on average 100% of the treated animals will develop one tumor).
If, however, the Tu complex is under the control of two regulatory
genes, the rate of simultaneous mutations of both of these regulatory
genes in 1 cell is 10 high-12, and the tumor incidence is 10 high-6.
This calculation shows that it is difficult to succeed in inducing
somatic mutationconditioned neoplasms if the Tu complex is controlled
by more than one regulatory gene. This calculation also suggests
that the insusceptibility of the animals of the purebred wild populations
is based on a polygenic system of regulatory genes directed against
cancer. Support for the assumption that the Tu complex of these
animals is controlled by only one regulatory gene comes from germinal
mutation-conditioned melanoma which occurred in the same genotype.
As a consequence of the inheritance of the mutation through the
germ line, the Tu complex becomes active in the developing progeny
as soon as the pigment cell precursors become competent for neoplastic
transformation. This process starts in the embryo and continues
in all areas of the developing fish where the pigment cell precursors
become competent, thus building a lethal "whole body melanoma,"
which reflects the genuine effect of the Tu complex on the pigment
cell system. It should be emphasized that the tumorous growths that
appear on germinal inherited melanoma (and other hereditary neoplasms),
i. e., both the mating-conditioned and the germ line mutation-conditioned
melanomas, are not due to the occurrence of somatic mutations during
development, because, in contrast to the somatic mutation-conditioned
tumors, the transformed cells always occur simultaneously in large
areas of the body and show permanent transformation and relapse
after complete removal. To study the molecular and biochemical background
of the somatic mutationconditioned melanomas we modified the experiment
that led to mating-conditioned spontaneously occurring melanomas
(see Fig. 8 and compare with Fig. 4). The Tu complex containing
platyfish chromosome was replaced by another which, instead of the
mutated dorsal fin
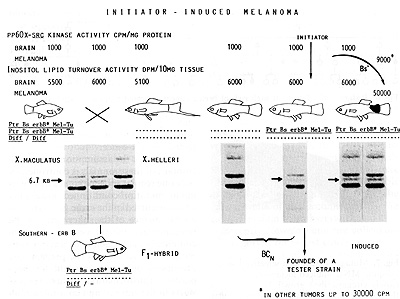
Fig.8. Crossing procedure for the production of mutagen-carcinogen-sensitive
backcross hybrids. Differences to the scheme shown in Fig. 3 are
the replacement of the mutated Dr' by the nonmutated body side-specific
regulatory gene Bs that suppresses melanoma formation. and the replacement
of the 4.9-kb EcoR1 fragment indicating x-erbB * by a 6.7-kb fragment
indicating the same x-erbB * gene. See text
specific regulatory gene Dl', contains the nonmutated body side-specific
regulatory gene Bs; in addition, the x-erb B* oncogene represented
by the 4.9-kb fragment was replaced by a translocated Y-chromosomal
copy that is represented by a 6.7-kb fragment. The other genetic
conditions are the same as those described in Fig. 4. Melanoma development
is suppressed by Bs in all purebred and hybrid animals carrying
the Tu complex. All BC hybrids carrying the Tu complex including
x-erbB* (they can be recognized by their pterinophore-specific reddish
coloration coded by Ptr) are susceptible to melanoma (and other
neoplasms) and may develop melanoma after treatment with physica]
or chemical carcinogens. Susceptibility to neoplasia or sensitivity
to carcinogens, respectively, is inherited in a Mendelian fashion,
but the tumors are, as a consequence of a somatic mutation of Bs
to Bs', nonhereditary and show no relapse after complete removal.
In contrast to the mating-conditioned spontaneous melanoma developing
BC hybrids the carcinogen-sensitive HC hybrids show no elevation
of pp60x-src activity as well as no elevation of inositol lipid
turnover in the brain. Elevations of these functions are only detected
in the neoplasm.
I. Nutrient- and Endocrine-Conditioned Neoplasia
Evidence for nutritional as well as endogenous and exogenous hormonal
influences on human cancer has been accumulating over the past 20
years [58]. The agents exerting these influences, of ten called
""promoters" or ""cocarcinogens,,' are by no means mutagenic carcinogens,
i. e., '"initiators," but appear as agents affecting the course
of differentation and the rate of proliferation of cells that have
already undergone the genetic key event underlying neoplasia irrespective
of whether they are tumor precursor cells or definite tumor cells;
the changes in cell differentiation and cell proliferation appear
as the last step in the chain of events resulting in cancer . Many
data on this subject come from epidemiological studies [59, 60].
It has been found that breast and colon cancer, which represent
a high percentage of total neoplasias in humans, are highly correlated
to animal tat intake in a large number of countries, and it has
been proposed that low animal fat intake is responsible for a low
incidence of these neoplasms, while high animal fat intake is responsible
for a high. incidence. The order of countries begins (low fat intake,
low tumor rate) with Thailand, the Philippines, Japan, Taiwan, continues
to Czechoslovakia, Austria, France, Switzerland, Poland, the Netherlands,
and Finland, and ends with the United States, Canada, Denmark, and
New Zealand (high fat intake, high tumor rate). A more critical
view, however, indicates that the tumor incidence of the Dutch is
twice as high as that of the Finns, though both have the same fat
intake. The same is true, if we compare the Swiss (high tumor incidence)
with the Poles (low tumor incidence, but same fat intake). The Danes
have an extremely high animal fat intake and an extremely high incidence
of breast cancer. If one compares, however , the population of Copenhagen
with that of the rural Denmark one finds that fat intake in Copenhagen
is much lower than in rural Denmark while urban Danes have a higher
tumor incidence than rural Danes. This is not to say that fat intake
will have no influence on the incidence of breast and colon cancer;
however, our critical view of the data makes clear that fat intake
alone cannot explain the differences in tumor incidence in different
countries. There could be genetic factors involved in such a way
that countries showing a high tumor incidence not only have a high
fat intake but also contain a high percentage of individuals that
are highly sensitive to the tumor-promoting effect of the fat. These
genetic factors may also be related to an effect on normal body
growth as has been reported in mouse studies [61, 62]. Thus, these
genes might interact with a multitude of other nutritional factors,
such as simple caloric intake, quantity and quality of protein ingested,
as well as drugs that influence the general condition of an individual.
Our own studies concentrated first on the construction of strains
of Xiphophorus that are highly sensitive to tumor promoters. Figure
9 shows the development of such a strain based upon the same genotypes
and crossing procedures as were used for the production of BC hybrids
that develop melanoma spontaneously (see fig. 4). The only difference
is that the genome of the animals contains a homozygous autosomal
gene, ""golden" (gig), by which pigment cell differentiation is
delayed in the stage of stem melanoblasts. Thus, the BC hybrids
corresponding to those developing malignant melanoma spontaneously
are incapable of developing a neoplasm. Chemical agents, such as
cyclic AMP , corticotropin, a large variety of steroid hormones
including testosterone, trenbolone [41 ], as well as general environmental
changes, such as decrease in temperature and increase in salinity
of the water in the tank, promote after a latent period of only
4 weeks (latent period of the carcinogen-dependent melanomas is
8 -12 months; see preceding paragraph) almost simultaneously the
differentiation of large amounts of the noncompetent cells to the
competent ones, which subsequently give rise to the melanoma exactly
at that place at the body of the fish where they are expected to
grow according to the basic crossing experiment (compare Figs. 4,
9).
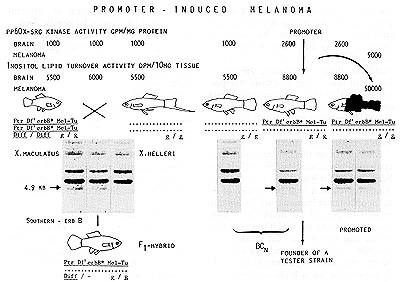
Fig. 9. Crossing procedure for the production of promoter-sensitive
backcross hybrids. The only difference to the scheme shown in Fig.
3 is the presence of the homozygous gene "golden", g/g, by which
pigment cell differentiation is delayed. See text
The very short latent period and the very fast growth of the occurring
melanomas as compared with that of the carcinogen-induced tumors
is remarkable, but is in line with the enhanced pp60x-src kinase
activity and the enhanced phosphoinositide turnover found in the
healthy tissues. It appears that, corresponding to the deletion
mutant El (see Fig.4), the molecular and biochemical machinery leading
to neoplasia is running in the susceptible but still tumorfree fish
and becomes immediately effective as the competent cells become
available for promotion of cell differentiation. The latter results,
again, indicate that both the enhanced activity of the xiphophorine
src oncogene and the enhanced phosphoinositide turnover are intimately
linked with the inheritance of x-erb B*, which is presumably involved
in the key signal preceding melanoma formation in Xiphophorus. They
furthermore show again that it should be possible to screen for
sensitivity and insensitivity to tumor promoters.
J. Future Goals
In this lecture I have tried to explain some observations on human
cancer from the view of a biologist working with a fish model. Of
course, what I have presented is not altogether new. Nevertheless,
what can we learn from the fish? First of all we should make informed
decisions to control the chemical and physical carcinogens and promoters
we receive today from our polluted environment. However, we should
keep in mind that cancer not only depends on the agents but also
on the genes that have been part of our evolution since life began.
These genes have experienced mutation, duplication, selection, and
genetic drift, and are controlled by oncostatic genes that keep
a tight rein on them. To learn how these regulatory genes keep the
oncogenes in check should be an challenging but fulfilling task
in the future of cancer research.
Acknowledgment
Thanks are due to Professor Steward Sell, Houston (Texas) and to
Prolessor Avril Woodhead, Brookhaven Natl. Lab. (New York), for
discussions and critical reading of the manuscript.
References
1. Kaiser HE (1981) Neoplasms -comparative pathology of growth
in animals, plants, and man Williams and Wilkins, Baltimore
2. Anders F (1983) The biology of an oncogene, based upon studies
on neoplasia. In. Neth R, Gallo RC, Greaves MF, Moore MAS, Winkler
K (eds) Modern trends in human leukemia V. Springer, Berlin Heidel
berg New York, pp 186-206 (Haematology and blood transfusion, vol
28.)
3. Anders F, Schartl M, Barnekow A, Schmidt CR, Lüke W, Jaellel-Dess
G, Anders A ( 1985) The genes that carcinogens act upon In: Neth
R, Gallo CR, Greaves MF, Janka G (eds) Modern trends in human leukemia
VI. Springer, Berlin Heidelberg New York, pp 228-252 (Haematology
and blood transfusion, vol 29.)
4. Burk O (1987) Konservierung voll homologen Sequenzen der kernproteinkodierten
Onkogene v-fos, v-myc und v-myb im Tierreich. Thesis, University
of Giessen
5. Kaiser P ( 1988) Restriktionsanalytische Untersuchungen der Konservierung
von homologen Sequenzen ZU den Onkogencn v-erb A, v-crb B and c-ncu
im Tierreich. Thesis, University of Giessen
6. Schleenbccker U ( 1988) Untersuchungen zur Struktur und Funktion
Voll homologen Sequenzen des Wachstumsfaktors und des Wachstumsfaktorrezeptors
bei Xiphophorus (Teleostei. Poeciliidae). Thesis, University of
Giessen
7. Zechel C (1988) Untersuchungen zur Struktur und Funktion v-crb
A- und v-crb B-homologer Sequenzen im XiphophorusTumorsystem. Thesis,
University of Giessen
8. Schmidt D (1988) Restriktionsanalytische Untersuchungen an genomischer
DNA Voll Xiphophorus. Thesis, University 0f Giessen
9. Gröger H (1987) Isolierung und Charakterisierung von c-myc-spezifischen
Klonell aus einer lambda EMBL 4 Genbank von Xiphophorus maculatus
( DrLi/Sr ) . Thesis, University 0f Giessen
10. Pfütz M (1988) Sequenzierung c-crb Aspezifischer Sequenzen aus
dem Genom voll Xiphophorus. Thesis, University 0f Giessen
11. DeFeo-Jones D, Scolnik E, Koller R, Dhar R (1983). ras-Related
gene sequences identified and isolated from Saccharomyccs cerevisiae.
Nature 306.707709 12. Pimentel E (1986) Oncogenes. CRC, Boca Ratoll
13. Reddy EP, Skalka AM, Currall T (1988) The oncogenc handbook.
Elscvicr, New York
14. Berridge MJ (1987) Insosit01 triphosphatc and diacylglyccr01.
TWO interacting second messengers. Allnu Rev Biochem 56.159-193
15 Berridge MJ (1987) Inosit011ipids and cell prolifcratioll Biochim
Biophys Acta 907.33-45
16. Berridge MJ, Irvine RF (1984) Inositol triphosphate, a novel
second messenger in cellular signal transduction. Nature 312.315-321
17. Schartl M, Barnekow A (1982) The expression in eukaryotes of
a tyrosine kinase which is reactive with pp 60v-srr antibodies.
Differentiation 23.109-113
18. Barnekow A, Schartl M ( 1983) Cellular src gene product detected
in the freshwater spongc Spongilla lacruslis. Mol Cell Biol 4:1179-1181
19. Albino AP, Strange R L, Oliff AL, Furth ME, Old L.J (1984) Transforming
ras genes from human melanoma: a manifestation of tumor heterogeneity.
Nature 308.69-72
20. Barnckow A, Paul E, Schartl M (1987) Expression of the c-sre
protooncogene in human skin tumors. Cancer Rcs 47.235-240
21. Ohno S (1970) Evolution by gene duplication Springer, Berlin
Hcidelberg New York
22. Zechel C, Schleenbecker U, Anders A, Anders F (1988) v-erbB
related sequences in Xiphophorus that map to melanoma determining
Mendelian loci and overexpress in a melanoma cell line Oncogene
3.605-617
23. Anders A, Anders F (1978) Etiology of neoplasia as studied in
the platyfish swordtail systeru. Eiochiru Eiophys Acta 516:61-95
24. Anders F ( 1967) Tumor formation in platyflsh swordtail hybrids
as a problcru of gene regulation. Experientia 23.1-10
25 Vielkind J, Dippel E (1984) Oncogcnc-related sequences in xiphophorine
fish prone to hereditary melanoma formation. Can J Genet Cytol 26:
607 -614
26. Anders F, Schartl M, Scholl E (1981) Evaluation of environmental
and hereditary factors in carcinogenesis, based on studies in Xiphophorus.
In. Dawe CD, Harshbarger JC, Kondo S, Sugimura T, Takayama S (eds)
Phyletic approaches to cancer. Japan Scientific Societies, Tokyo,
pp 289-309
27. Gordon M (1947) Speciation in fishes. Adv Genet 1.95-132
28. Kallman KD (1975) The platyfish, Xipho rus maculatus. Handb
Genet 4: 81 -132
29. Anders F, Schartl M, Earnekow A, Anders A ( 1984) Xiphophorus
as a in vivo model for studies on normal and defective control of
oncogenes. Adv Cancer Res 42.191-275
30. Moriwaki K, Shiroishi T, Miyashita N, Kondo N, Imai H (1980)
Intersubspecies hybrid of mice as a tool for studying genetic governing
tumor development Gann Monogr 25.165 176
31. Anders F (1981) Erb- und Umweltfaktoren im Ursachengefüge des
neoplastischen Wachstums nach Studien an Xiphophorus Verhandl Ges
Dtsch Naturforscher Ärzte 22. 106-119
32. O'Erien S (1980) The extent and character of biochemical genetic
variation in the domestic cat. J Hered 71.2-8
33. Fuerst PA, Chakraborty R, Nei M (1977) Statistical studies on
protein polymorphism in natural populations I. Distribution ofsinglc
locus hetcrozygosity. Genetics 86.455-483
34. Schull W J (1979) Genetic structure of human populations. J
Toxicol Environ Health 5 17-25
35. Lubs IIA, Kimberling WJ, Hecht F, Patil SR, Brown J, Gerald
P, Summit RL (1977) Racial differences in the frequency of Q and
G chromosomal heteromorphisms. Nature 268.631-632
36. Yamada K, Hasegawa T ( 1978) Types and frequencies of Q-variant
chromosomes in a Japanese population. Hum Genet 44. 89-98
37. Higginson J (1969) Present trends in cancer epidemiology. Proc
8th Canadian Cancer Conf. Pergamon, Toronto, pp 4075
38. I Iigginson J ( 1988) Changing concepts in cancer prevention.
limitations and implications for future research in environmental
carcinogcnesis. Cancer Res 48. 1383 1389
39. Iloover K (1984) Use of small fish species in carcinogenicity
testing. NCI Monogr 65
40. Zechel C, Schleenbecker U, Anders A, Anders F (1988) Regulation
of gene expression in the Gordon-Kosswig mclanoma system. In. Schröder
H (ed) Genetics and mutagenesis of fish. (in press)
41. Montesano R, Earth H, Vainio H, Wilborn J, Yamasaki H (1986)
Long-term and short -term assays for carcinogenesis. IARC scientifIc
publications no 83. IARC, Lyon, pp 103-126
42. Mawdesley- Thomas LE (1975) Neoplasia in fish. In. Tibelin WE,
Migaki G (eds) The pathology of fishes. University of Wisconsin
Press, pp 805-870
43. Dahme E, Weiss E ( 1988) Grundriß der speziellen pathologischen
Anatomie der Haustiere, 4th edn. Enke, Stuttgart
44. Mäueler W (1988) Untersuchungen zur tumorspezifischcn Genexpression
bei Xiphophorus (Teleostei. Poeciliidae). 1. Enzyme des Intermediärstoffwechsels,
2. Expression von Proto-Onkogenen. Thesis, University of Giessen
45. Krekeler G (1988) Isolierung und Charakterisierung v-myb-homologer
Sequenzen aus einer Genbank von Xiphophorus (Pisces. Poeciliidae).
Thesis, University of Giessen
46. Schartl M, Schmidt CR, Anders A, Eamekow A ( 1985) Elevated
expression of the cellular src gene of differing etiologies in Xiphophorus.
lnt J Cancer 36: 199207
47. Gronau T (1987) Untersuchungen zur Organisation, Aktivität und
Wirkung des zellulären Onkogens c-src iru Xiphophorus- Tumorsystem.
Thesis, University of Giessen
48. Pröfrock A (1988) Untersuchungen zuru Phosphatidylinosit- Tumovcr
an ausgewählten Xiphophorus-Genotypen. Thesis, University of Gicssen
49. Smith AD, Gronau T, Pröfrock A, Zcchcl C, Bird IM, Lane PA,
Earnekow A, Anders A, Anders F ( 1988) EG F receptor gene, inositol
lipid turnover and C-src activity in key processes preceding melanoma
in Xiphophorus. In. Lynch HT, Fusaro RM (eds) Hereditary Malignant
Melanoma, CRC, Boca Raton (in press)
50. lynch HT, Lynch JF, Fusaro RM (1985) Clinical importance of
familial cancer, In' Müller H, Weber W, Kuttapa T (eds) Familial
cancer, Karger, Basel, pp 6-12
51. Ahu,ja MR, Lepper K, Anders F (1979) Sex chromosome aberrations
involving loss and translocation of tumor-indueing loci in Xiphuphurus,
Experientia 35'28-29
52. Anders A, Anders F, Klinke K (1973) Regulation of gene expression
in the Gordon-Kosswig melanoma system, In' Schröder H (ed) Genetics
and mutagenesis of fish, Springer, Berlin Heidelberg New York, pp
33-63
53., Anders A, Dess G, Nishimura S, Kersten H (1985) A molecular
approach to the study of malignancy and benignancy in melanoma of
Xiphuphuru,s, In' Bagnara J Klaus SN, Paul E, Schartl M (eds) Pigment
cell 1985 University of Tokyo Press, pp 315-324
54. Müller HJ Weber W, Kuttapa T (1985) Familial cancer, Karger,
Basel
55. Rudolph B, 1Harbott J Lampert F (1988) Fragile sites and neuroblastoma'
Fragile site at one p 13,1 and other points on lymphocyte chromosomes
from patients and family members, Caneer Genet Cytogenet 31'83-94
56. Lyneh HT, Lynch JF (1985) Genetics and eolorectal cancer, In'
Müller HJ Weber W, Kuttapa T (eds) Familial cancer, Karger, Easel
57. Newman E, Austin MA, Lee M, King MC (1988) Inheritance of human
breast cancer' Evidence for autosomal dominant transmission in high-risk
families, Proe Natl Aead Sci USA 85'3044-3048
58. Cohen LA (1987) Diet and cancer Sci Am 257 (5)42-48
59. Hecker E, Fusenig NE, Kunz W, Marks F, Thielmann HW ( 1982)
Cocarcinogenesis and biological effects of tumor promoters, Raven,
New York
60. Carroll KK (1975) Experimental evidence of dietary factors and
hormone-dependent cancers Cancer Res 35: 3374-3383
61 Wynder EL (1975) The epidemiology of large bowel cancer, Cancer
Res 35'3388-3394
62. Heston WE (1957) Effects of genes located on chromosomes III,
V, VII, IX, and XIV on the occurrence of pulmonary tumors in the
mouse, Proc Intern Genetics Symposia, 1956 Cytologica [Suppl]' pp
219-224
|












