|
*Clinical investigations at Stanford University Medical Center described in this article were supported by research grant CA-OSR38 from the National Cancer Institute, National Institutes of Health, U.S. Department of Health, Education, and Welfare, The collaborative assistance of a multidisciplinary team of colleagues is gratefully acknowledged
The nature of Hodgkin's disease has been the subject of more than 100 years of intense debate, The occurrence of massive lymphadenopathy, with later spread to the lungs, liver, bone marrow, and other tissues, and the inevitably fatal course of the disease suggested to many scholars that it was a form of malignant neoplasm, Others, however, impressed with its frequently febrile course, with the occasional waxing and waning in size of enlarged lymph nodes, and with the frequent coexistence of tuberculosis or other infectious diseases at autopsy, considered it some form of granulomatous infection or inflammation, Finally, as awareness has grown concerning the curious defect of immune responsiveness which occurs so often in Hodgkin's disease, a third hypothesis has been put forward suggesting that it may stem from a chronic immunologic disorder, Certain similarities to the histologic features seen in immunologic reactions of the graft-vs-host type led Kaplan and Smithers ( 1959) to suggest that Hodgkin's disease might represent an autoimmune process involving an interaction between neoplastic and normal lymphoid cells, a hypothesis later extended and developed by others (De Vita 1973; Green et al, 1960 ; Order and Hellman 1972 ), Definitive evidence that Hodgkin's disease is indeed a malignant neoplasm, albeit a remarkably atypical one, finally emerged during the last two decades from cytogenetic and cell culture studies which demonstrated that the giant cells of Hodgkin's disease satisfy two of the most fundamental attributes of neoplasia: aneuploidy and clonal derivation,
It was once considered that the giant binucleate or multinucleate
Reed-Sternberg cells most closely resembled and were therefore probably
derived from the histiocyte (Rappaport 1966), However, histochemical
studies (Dorfman 1961 ) failed to reveal the presence of nonspecific
esterase, an enzyme characteristically present in cells of the monocyte-histiocytemacrophage
series, Meanwhile, growing awareness of the remarkable changes in
size and morphology which small lymphocytes may undergo during the
process of lymphobJastoid transformation in response to lectins
and specific antigens led to the hypothesis that the Reed-Sternberg
cell might be an unusual form of transformed lymphocyte (Dorfman
et al, 1973; Taylor 1976), There has also been disagreement as to
whether Reed-Sternberg cells are capable of DNA synthesis and mitosis,
Although giant mitotic figures have been observed by some investigators,
cells arrested in mitosis by treatment with vinblastine appeared
to be limited to the mononuclear cell population in other studies
(Marmont and Damasio 1967), After short-term incubation of cell
suspensions of fresh lymph node biopsies from ten patients with
Hodgkin's disease, autoradiographic evidence of incorporation of
tritiated thymidine into DNA was seen only in mononuclear cells
(Peckham and Cooper 1969), suggesting that the mononuclear Hodgkin's
cells are the actively proliferating neoplastic cells and that the
Reed-Sternberg cells are nonproliferating, end-stage, degenerative
forms. Later studies, however, were more successful in revealing
labeling in Reed-Sternberg cells, as were cell culture studies by
Kadin and Asbury (1973 ) and by Kaplan and Gartner (1977). In the
last-cited report, it was observed that 17 (20.7 %) of 82 binucleate
or multinucleate giant cells were labeled (Fig. 1 ), a proportion
only moderately Jess than that observed among the mononuclear cell
population (334 of 918, or 36.5 c/c ). Moreover, binucleate mitotic
figures could be seen in some cells of the same culture. Accordingly,
it is now clear that Reed-Sternberg cells are indeed capable of
DNA synthesis and mitotic division and may thus be considered, together
with their mononuclear counterparts, to be the neoplastic cells
of Hodgkin's disease. Chromosome studies have been carried out by
the direct method or following short-term incubation of tissues
involved by Hodgkin's disease in at least lOO cases from 1962 through

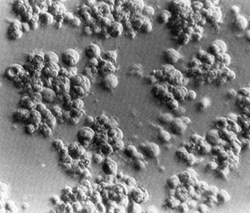 Fig. 2. Long-term culture of cells from the involved spleen of a patient with Hodgkin's disease. Note the clusters of adherent giant cells. The huge size of these cells may be appreciated by comparison with that of the occasional lymphocytes still persisting 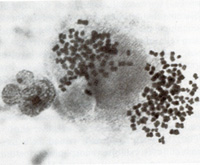
Lymphangiography swept away earlier misconceptions concerning the
unpredictable, capricious distribution of lymph node involvement
in patients with Hodgkin's disease and made possible systematic
attempts to map sites of disease, Rosenberg and Kaplan (1966), in
a study of lOO consecutive, previously untreated patients with Hodgkin's
disease, found that involvement of various chains of lymph nodes
was distinctly nonrandom; when a given chain of lymph nodes was
affected, other chains known to be directly connected with it via
lymphatic channels were likely also to be involved, either concurrently
or at the time of first relapse, Even extralymphatic sites such
as the lung, liver, and bone marrow were more likely to be involved
in association with certain predictable patterns of lymph node and/or
spleen involvement. These studies were subsequently extended (Kaplan
1970, 1980) to overlapping series of 340 and 426 consecutive previously
untreated cases, with results which strongly confirmed and reinforced
the initial conclusions. Similar analyses have been presented by
other groups of investigators (Banfi et al. 1969; Han and Stutzman
1967), again with generally similar conclusions. Two distinctivcly
liifferent theories, the '.contiguity" theory of Rosenberg and Kaplan
( 1966) and the "susceptibility'. theory of Smithers ( 1970, 1973),
have been proposed to account for the patterns of spread observed
in Hodgkin's disease. The contiguity theory postulates that Hodgkin's
disease is a monoclonal neoplasm of unifocal origin which spreads
secondarily by metastasis of pre-existing tumor cells, much like
other neoplasms, except that the spread is predominantly via lymphatic
rather than blood vascular channels. The term contiguity refers
to the existence of direct connections between pairs of lymph node
chains by way of lymphatic channels which do not have to pass through
and be filtered by intervening lymph node or other lymphatic tissue
barriers. Smithers (1973) suggested that the giant cells of Hodgkin's
disease may move in and out of lymph nodes from the blood stream,
following a traffic pattern similar to that known to occur with
normallymphocytes. Emphasis was placed on the concept that Hodgkin's
disease is a systemic disorder of the entire lymphatic system. Thus,
the possibility was suggested that the disease may have a multi
focal origin, perhaps by spread of a causative agent with de novo
reinduction in different sites rather than the spread of pre-existing
tumor cells. After an initial site had become involved, the theory
predicted that each of the remaining lymph node chains would have
an independent probability of next becoming involved which was assumed
to be proportional to the probabilities of initial involvement of
the corresponding lymph node chains in patients with Stage I disease.
Careful mapping of the initial sites of involvement in consecutive,
previously untreated patients revealed the occurrence of noncontiguous
patterns in only 4 (2%) of 185 patients with Stage 11 disease (Kaplan
1970). Hutchison ( 1972) compared the observed distributions in
158 of our Rye Stage 11 cases whose calculated frequencies were
based on the random association of two or more sites with the probabilities
given by their respective frequencies in 53 observed Stage 1 cases.
The observed patterns for two or three involved sites departed significantly
from random expectation. In particular, there was an apparent deficiency
of bilateral cervical node involvement in the absence of associated
mediastinal lymphadenopathy, an excess frequency of association
between cervical and mediastinal node involvement, and a marked
deficiency of all noncontiguous contralateral distributions. Lillicrap
(1973) compared the predictions of the Smithers susceptibility hypothesis
with the observed patterns of spread in three different series of
patients with Hodgkin's disease. Bilateral cervical lymph node disease
was observed significantly less often than predicted, whereas involvement
of the neck and mediastinum was more frequent than predicted. There
were 46 instances of homolaterai cervical-axillary involvement and
only two contralateral cases, whereas equal numbers of each would
have been predicted by susceptibility theory. Conversely, the observed
patterns were consistent with the contiguity theory in all but 8
( 4%) of 212 cases. Modifications of the susceptibility theory were
subsequently proposed by Smithers et al. ( 1974) in an attempt to
make the theory more consistent with observed distribution frequencies.
These modifications, which accept the concept of spread via lymphatic
channels, exhibit appreciably better agreement with observed patterns
of two and three sites of involvement. The contiguity theory has
also been tested with respect to the sites of first relapse in patients
with regionally localized disease treated with limited field radiotherapy.
Rosenberg and Kaplan (1966) found that 22 of 26 extensions of disease
were to contiguous lymph node chains. Similar findings have been
reported by others (Banfi et al. 1969; Han and Stutzman 1967). The
most controversial issue is the association between involvement
of the lower cervical-supraclavicular lymph nodes and the subsequent
occurrence of relapse in the upper lumbar para-aortic nodes. Among
80 such cases at risk, Kaplan ( 1970) observed para-aortic node
extensions in 29 (360/0 ). This was the single most prevalent site
of extension in patients treated initially with local or limited
field, supradiaphragmatic radiotherapy. Transdiaphragmatic extension
was also the first manifestation of relapse in 33 ( 40% ) of 83
patients with clinical Stage land 11 disease studied by Rubin et
al. (1974 ). Many para-aortic lymph node relapses occurred several
years after initial treatment and frequently involved lymph nodes
which were well visualized and appeared normal on the originallymphangiogram.
It was suggested (Kaplan 1970; Rosen berg and Kaplan 1966) that
spread in these instances had occurred in the retrograde direction
Unresponsiveness to tuberculin was the first immunologic abnormaJity
observed in patients with Hodgkin's disease. Dorothy Reed ( 1902
reportet that tuberculin was given in five cases but without reaction."
However, the immunologic deficiency is not specifically re stricted
to tuberculosis. Schier et al. ( 1956) tested the capacity of patients
with Hodgkin's disease to mount delayed hypersensitivity re actions
to a diversified battery of natural antigens and found that most
were unresponsive to all of the antigens tested. Unfortunately,
the significance of the early studies cannot be assessed because
many patients had been treated, and none had been staged by modern
methods. A series of 50 previously untreated patients with Hodgkin's
disease, all staged with the aid of lymphangiography and other modern
diagnostic procedures, was studied at the National Cancer Institute
by Brown et al. (1967). Responsiveness to the five antigens tested
was impaired relative to controls. However, reactions in eight patients
with clinical Stage I Hodgkin's disease appeared to be comparable
with those of normal controls. With increasing clinical stage, responsiveness
decreased sharply. Positive responses to one or more intradermal
antigens were noted in seven of eight patients with Stage I disease,
13 of 24 in Stage II, three of seven in Stage III, and 5 of 11 in
Stage IV. These studies were later extended to a total of 103 patients
with previously untreated disease with generally similar results
(Young et al. 1972). Only seven patients, all of whom had constitutional
symptoms, were completely anergic (unresponsive to all tests). Among
a total of 185 patients studied at Stanford University Medical Center
from 1964 through 1968 there were 28 patients with previously untreated
Stage 1 disease, of whom only 12 ( 43% ) responded to mumps antigen
and few responded to any other cutaneous antigen (Kaplan 1970).
A second study initiated in 1969 accrued 154 previously untreated
patients, all staged with the aid of lymphangiography and laparotomy
with splenectomy (EItringhall and Kapliin 1973). Only 51 of 151
evaluable patients (34%) responded to one or more intradermal antigens,
and a positive reaction to mumps antigen was observed in only 40
(25 %) of 151 patients. There was no significant influence of clinical
stage on response to mumps antigen. In contrast to the observations
of the Bethesda group, unresponsiveness did not occur more frequently
among patients with constitutional symptoms. In tests with streptokinase-streptodornase
(SK-SD), only 6 (10%) of 58 untreated patients with Hodgkin's disease
reacted to 5 units, whereas 93 % of age -and sex-matched controls
were known to respond to the same dose level (Eltringhall and Kaplan
1973 ). Clinical investigations using chemical agents known to have
the property of inducing delayed cutaneous hypersensitivity reactions
essentially indistinguishable from those induced by tuberculin have
the advantage that the fact of exposure to the agent and the timing
of that exposure are both under the control of the investigator.
The most extensively used of these chemicals is 2,4-dinitrochlorobenzene
(DNCB). In a series of 50 untreated patients, Brown et al. (1967)
observed positive responses in 35 (70%) to sensitization with DNCB
at a concentration of 2.0%. Impressed by the fact that all eight
of their patients with Stage I disease reacted positively to DNCB
and that seven of the eight reacted to at least one intradermal
antigen, the Bethesda group concluded that the development of anergy
is probably a secondarily acquired manifestation associated with
advancing anatomic extent of involvement rather than an intrinsic
component of the pathogenesis of Hodgkin's disease. In an initial
study involving 185 previously untreated patients sensitized with
2.0%( DNCB at Stanford University Medical Center from 1964 through
1968, an extremely high incidence of anergy was observed, even in
patients with Stage I disease (Kaplan 1970). De Gast et al. (1975)
also observed negative reactions to challenge after sensitization
with the same concentration of DNCB in 20 of 30 patients (67 %),
including two of five with Stage I disease, and Case et al. (1976)
reported negative reactions in 24 of 50 patients ( 48% ), including
three of eight with Stage I disease. In a subsequent Stanford study
involving untreated patients staged routinely with lymphangiography
and laparotomy with splenectomy, three different sensitizing concentrations
of DNCB (0.1,0.5, and 2.0%) were used (Eltringham and Kaplan 1973).
Sensitization and challenge with DNCB occurred prior to the initiation
of treatment. At a sensitizing concentration of 0.5 %, only 10 (26%
) of 39 patients responded as compared with 83% of normal controls.
This study was ultimately extended to encompass a total of 531 previously
untreated patients of all stages (Kaplan 1980). There were 113 positive
responses (36.3 %) among 311 patients with Stage I and II disease,
a response rate only slightly greater than that among patients with
Stage III and IV disease (56 of 220, or 25.5 % ). Of a total of
355 asymptomatic patients, 128 (36.1% ) responded, a significantly
higher response rate than that of patients with constitutional symptoms
( 41 of 176, or 23.3% ). These data support the conclusion that
cell -mediated immune reactivity is indeed impaired in patients
with Hodgkin's disease. However, the impairment is not an all-or-none
phenomenon but a more subtile continuous gradient of immunologic
dcficit which is present in some degree even in patients with the
earliest manifestations of the disease. A number of in vitro tests
are considered analogs of ccll-mediated immunc responses. These
include the capacity of lymphocytes to: ( 1) undergo lymphoblastoid
transformation after stimulation by lectins or antigens and to respond
in thc mixcd lymphocyte reaction, (2) to bind sheep erythrocytes
to their surface membranes (E-rosette formation), and (3) to bind
and become agglutinated by certain lectins and to mediatc the polar
migration ( capping) and shedding of the bound lectins from the
cell membranc. Brown et al. ( 1967) noted a mean lymphocyte response
to phytohemagglutinin (PHA) of 49% in 43 patients with untreated
Hodgkin's disease, a highly significant decrease from the 72% mean
valuc observed in their controls. However, responses in patients
with Stage I disease were within the normal range. Very similar
responses to PHA were noted by De Gast et al. ( 1975 ) in a series
of 30 patients with Hodgkin's disease. However, these investigators
noted that lymphocyte stimulation by a-hemocyanin was impaired in
11 of 15 patients and that the DNCB skin test reaction was also
negative in 10 of the 11 nonresponsive individuals. Lymphoblastoid
responses to another antigen, tctanus toxoid, were negativc in six
of nine patients studied by Fuks ct al. (1976a). Gaines et al. (1973)
observed that lymphocytes from three patients with positive Toxopla'ima
dye tcst titers as well as those of 20 with negative titers failed
to respond to Toxoplasma antigen in vitro. Responses to SK-SD were
also negative in 22 of 23 untreated patients. Holm et al. (1976)
in a study of 31 patients with Hodgkin's disease noted that only
1 of 12 skin test positive patients had an impaired lymphocyte response
to the antigen in vitro ; conversely, only 1 of 19 patients with
a negative skin test reaction had a normallymphoblastoid response
to tuberculin (PPD) in vitro. Deficient responses to PPD were observed
in 7 (47%) of 15 patients with Stage for II disease and in 11 (55%)
of 20 patients with Stage III or IV disease. Modifications of technique
succeeded in revealing unambiguous abnormalities of the PHA stimulation
response even in patients with Stage I disease. Matchett et al.
( 1973 ) noted good initial responses during the first 2 days in
patients with localized disease, but these responses were not sustained
at 4 or 5 days. When the daily uptake of tritiatcd thymidine (3H-
TdR) by limiting concentrations of cells was used as the index of
response, all of 26 patients, including those with localized disease
and no symptoms, showed a striking degree of abnormality. Levy and
Kaplan ( 1974 ) measured the uptake of tritiated leucine (3H-Leu)
into protein in peripheral blood lymphocytes stimulated with a range
of PHA concentrations. This assay requires only 20 h for completion,
so that cell viability can be preserved in the absence of serum,
thus enhancing precision and reproducibility. They studied 37 normal
subjects and 44 consecutive untreated patients with Hodgkin's disease,
all staged with lymphangiography, bone marrow biopsy, and in those
with negative marrow biopsies, laparotomy with splenectomy. The
peak response of normal donor lymphocytes was noted at a PHA concentration
of 1 ,ug/ml. The response of lymphocytes from patients was very
significantly below normal at all but the highest PHA concentrations
tested. The impairment of response was observed both in patients
with limited (Stage I and II) as well as those with advanced (Stage
111 and IV) disease. These results remained essentially unchanged
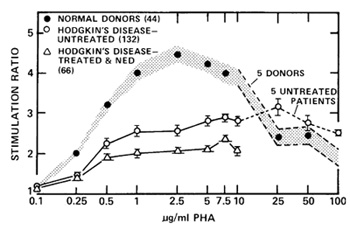
after this study had been extended (Fuks et al. 1976a) to include
132 patients with untreated Hodgkin's disease (Fig. 5). Stimulation
by another lectin, concanavalin A (Con A), revealed impaired responses
in a series of 18 patients. Concentration-dependent defects in lymphocyte
response to PHA were also observed by Ziegler et al. (1975) and
by Faguet ( 1975 ) in untreated patients with various stages of
Hodgkin's disease. Negativc mixed lymphocytc reactions (MLR) were
observed by Lang et al. ( 1972) in 7 (22% ) of 32 patients with
untreated Hodgkin's disease. In a study of 30 patients, Ruhl et
al. (1975) found that the capacity of lymphocytes from patients
with Hodgkin's disease to respond to allogeneic cells was significantly
Aisenberg AD, Weitzman S, Wilkcs B (1978) Lymphocyte rcccptors for concanavalin A in Hodg kin's disease. Blood 51-439-443- Banfi A, Bona donna G, Carnevali G, Fossati-Bellani F (1969) Malignant Iymphomas- further studies on their preferential sites of involvement and possible modc of sprcad. Lymphology 2 -130-138- Ben-Bassat H, Goldblum N (1975) Concanavalin A receptors on the surface mcmbrane of lymphocytes from patients with Hodgkin-s disease and other malignant lympho mas Proc Natl Acad Sci USA 72 1046-1049 Bieber MM, Fuks Z, Kaplan HS (1975) E-rosette inhibiting substance in Hodgkin's diseasc spleen extracts. Clin Exp Immunol 29-369-375- Bieber MM- King DP, Strober S- Kaplan HS (1979) Characterization of an E-rosette inhibitor (ERI) in the serum of paticnts with Hodgkin-s discase as a glycolipid Clin Res 27 .S1A- BjorkholmM, Holm G- Mellstedt H, Pettcrsson D (1975) Immunological capacity of Iymphocytcs from untreatcd patients with Hodgkin-s disease cvaluated in mixed lymphocyte culture. Clin Exp ImmunoI22:373-377 -Bobrove AM, Fuks Z, Strober S- Kaplan HS (1975) Quantitation of T- and B-Iymphocytes and cellular immune function in Hodgkin-s disea5e- Cancer 36 169-179 -Brown RS, Haynes HA, Foley HT, Godwin HA- Berard CW, Carbone PP- Immunologic, clinical and histolog.ic featurcs of 50 untreatcd patients- Ann Intern Med 67-291-302- Browse !';L- Lord RSA, Taylor A ( 1971) Pressure waves and gradients in the canine thoracic duct. J physiol (Lond) 213.507-524- Carr I (1975) The ultrastructure of the abnormal reticulum cells in Hodgkin's disease. J Pathol 115:45-50- Case DC Jr, Hansen JA, Corralcs E, Young CW, DuPont B, Pinsky CM, Good RA (1976) Comparison of multiple in vivo and in vitro parameters in untreated patients with Hodgkin's disease- Cancer 38-1S07-1815 -De Gast GC, Halic MR, Nieweg HO ( 1975) Immunological responsivencss against two primary antigens in untreated patients with Hodgkin's disease Eur J Cancer 11 217-224- De Vita VT (1973) Lymphocyte reactivity in Hodgkin's disease a lymphocytc civil war:\ Engl J Med 289. 801-S02 Dorfman RF ( j 961) Enzymc histoche mistry of the cclls in Hodgkin's disease and allied disorders. !';ature 190 925-926 -Dorfman RF, Rice DF, Mitchcll AD, Kempson RI, Levinc G ( 1973) Ultrastructural studies of Hodgkin's disea se. Natl Canccr Inst Monogr 36221-23S -Dumont AE, Martelli AB ( 1973) Expcrimental studics bearing on thc question of rctrograde spread of Hodgkin's discase via thc thoracic duct. Cancer Res 33-3195-3202- Eltringham JR, Kaplan HS (1973) Impaired dclayed hypersensitivity rcsponses in 154 patients with untreatcd Hodgkin's disease. Natl Cancer Inst Monogr 36.107-115 Engeset A, Brennhovd 10, Christensen I, Hagen S, Host H, Liverud K, Nesheim A ( I 96S) Sternberg-Reed cells in the throacic duct lymph of patients with Hodgkin'5 disease- A preliminary report- Cytologic studies in connection with lymphography. Blood 31 99-103 -Englcman EG, Benike C, Hoppe R, Kaplan HS (1979) Suppressor cells of the mixed lymphocyte rcaction in patients with Hodgkin's disease. Transplant Proc 11 .lS27-1829 -Engleman EG, Benike C, Hoppe RT, Kaplan HS, Berberich RT ( 1980) Autologous mixcd lymphocyte reaction in patients with Hodgkin's disease- evidcnce for a T cell defect J Clin Invest 66149-15S Faguet GB (1975) Quantitation of immunocompetencc in Hodgkin's di5ease. J Clin Inve5t 56.951-957 -Fuks Z, Strober S, Bobrove AM, Sasazuki T, McMichael A, Kaplan HS (1976a) Long-term cffects of radiation on T- and B-Iymphocytes in peripheral blood of patients with Hodgkin.s disease. J Clin Invcst 58 -803-S j 4 -Fuks Z, Strober S, Kaplan HS ( 1976b ) Interaction betwecn serum factors and T -lymphocytes in Hodgkin'5 disease. N Engl J. Med 2951273-1278- Fuks Z, Strober S, King DP, Kaplan HS ( 1976c) Reversal of cell surface abnormalities of T -lymphocytes in Hodgkin's disease after in vitro incubation in fetal sera. J Immunol 117.1331-1335 -Gaines JD, Gilmer MA, Remington JS (1973) Deficiency of lymphocyte antigen recognition in Hodgkin's disea se. NatlCancer InstMonogr36. I 17-121-Gallmeicr WM, Boecker WR, Bruntsch U, Hossfeld DK, Schmidt CG ( 1977) Characterization of a human Hodgkin cell line and a lymphoblastic EBNA-negative cell line dcrived from a non-Hodgkin's lymphoma paticnt Hacmotol Bluttransfus 20.277-281 Garvin AJ, Spicer SS, Parmley RT, Munster AM ( 1974) Immunohistochemical demonstration of 19G in Reed-Sternberg and other cells in Hodgkin's disease. J Exp Med 139- 1077-1083 -Gearhart PJ, Sigal !';H, Klinman NR ( 1975) Production of antibodies of identical idiotype but diverse immunoglobulin classes by cells derived from a single stimulated B cell Proc Natl Acad Sci USA 72-1707-1711 -Goodwin JS, Messner RP, Bankhurst AD, Peake GT, Saiki JH, Williams RC Jr ( 1977) ProstagJandinproducing suppressor cells in Hodgkin's disease N Engl J Med 297. 963-96ij Green I, Inkelas M, Allen LB (1960) Hodgkin's diseasc .a maternal-tofoetal lymphocyte chimacra ') Lancct 1 30-32- Grifoni V, Del Giacco GS, Tognella S, Manconi PE, Mantovani G ( 1970) Lymphocytotoxins in Hodgkin's disease Ital J Immunol Immunopathol 21-31 Han T, Stutzman L (1967) Mode of spread in patients with localized malignant lymphomas Arch Intern Med 120. 1-7 Hillinger SM, Herzig GP ( 1978) Impaired ccll-mediatcd immunity in Hodgkin's disease mediated by suppressor Iymphocytcs and monocytes. J Clin Invest 61 1620-1627- Holm G, Mellstcdt H, Bjiirkholm M, Johansson B, Killander D, Sundblad R, Siderberg G ( 1976) Lymphocyte abnormalities in untreated patients with Hodgkin's disease. Cancer 37751-762 Hutchison GB (1972) Anatomic patterns by histologic type of localized Hodgkin's disease of the upper torso Lymphology 5.1-14 Kadin ME, Asbury AK ( 1973) Long-term culturcs of Hodgkin's tissue. A morphologic and radioautographic study. Lab Invest 2ij .181-184 ~ Kadin ME, Stites DP, Levy R, Warnke R ( 1978) Exogenous origin of immunoglobulin in Recd-Sternbcrg cells of Hodgkin's discase N Engl J Mcd 299' 1208-1214 -Kaplan HS (1970) On the natural history, treatment, and prognosis of Hodgkin's disease Harvey Lecturcs, 196ij-1 969, Academic Prcss New York pp 215-259- Kaplan HS (1980) Hodgkin's Disease, 2nd ed Harvard University Press, Cambridge, MA -Kaplan HS, Gartner S (1977) "Sternberg-Rccd" giant cells of Hodgkin's disease. cultivation in vitro, heterotransplantation, and characterization as neoplastic macrophages Int J Canccr 19 511-525 Kaplan HS, Smithers OW (1959) Auto-immunity in man and homologous disease in mice in relation to the malignant lymphomas. Lancet 2 1-4 -Kirschner RH, Abt AB, O'Connell MJ, Sklansky BD, Grccne WH, Wiernik PH ( 1974) Vascular invasion and hematogenous dissemination of Hodgkin's disease. Cancer 341159-1162- Lamoureux KB, Jaffe ES, Berard CW, Johnson RE (1973) Lack of identifiable vascular invasion in patients with extranodal dissemination of Hodgkin's disease. Cancer 31'824-825- Lang JM, Oberling F, Tongio M, Maycr S, Waitz R ( 1972) Mixed lymphocyte reaction as assay for immunological competence of lymphocytes from patients with Hodgkin's disease Lancet l' 1261-1263 -Lang JM, Bigel P, Oberling F, Maycr S (1977) Normal active rosette-forming cells in untreated patients with Hodgkin's disease Biomedicine 27. 322-324 -Levy RA, Kaplan HS ( 1974 ) Impaired lymphocyte function in untrcated Hodgkin's disease N Engl J Med 290181-1ij6 -Lillicrap SC ( 1973) Modes of spread of Hodgkin's disease. Br J Radiol46 I ij-23 -Long JC, Zamecnik PC, Aisenberg AC, Atkins L ( 1977) Tissue culture studies in Hodgkin's disease Morphologic, cytogenetic, cell surface and enzymatic properties of cultures derived from splenic tumors J Exp Mcd 145.14ij1-1500 -Marmont AM, Oamasio EE ( 1967) The effects of two alkaloids derived from Vinca Rosea on the malignant cells in Hodgkin's disease, lymphosarcoma and acute leukemia in vivo. Blood 291-21- Matchett KM, Huang AT, Kremer WB ( 1973) 1mpaired lymphocyte transformation in Hodgkin's disease. Evidence for depletion of circulating T-Iymphocytes J Clin Invest 52.1908-1917 -Mintz U, Sachs L ( 1975) Membrane differences in peripheral blood Iymphocytcs from patients with chronic lymphocytic leukemia and Hodgkin's disease Proc Natl Acad Sci USA 72 2428-2432- Moroz C, Lahat M, Biniaminov M, Ramot B ( 1977) Ferritin on the surface of lymphocytes in Hodgkin's disease patient A possible blocking substance removed by levamisole Clin Exp Immunol29 30-35- Naeim F, Waisman J, Coulson WF ( 1974) Hodgkin's discase . the significancc of vascular invasion Cancer 34.655-662 -Neyazaki T, Kupic EA, Marshall WJ, Abrams HL ( 1965) Collateral Iymphatico-venous communication after experimental obstruction of the thoracic duct. Radiology 85.423-431- Order SE, Hellman S ( 1972) Pathogenesis of Hodgkin's disease Lancet 1.571-573 Peckham MJ, Cooper EH ( 1969) Proliferation characteristics of thc various classcs of cells in Hodgkin's disease Cancer 24135-146 Rappaport H (1966) Tumors of the hematopoietic system. Atlas of tumor pathology, Sect III, rasc 8 Armcd Forces Institute of Pathology, Washington, DC -Rappaport H, Strum SB (1970) Vascular invasion in Hodgkin's disease. its incidence and relationship to the spread of the disease. Cancer 25'1304-1313 -Rced DM ( 1902) On the pathological changes in Hodgkin's disease, with special reference to its relation to tuberculosis Johns Hopkins Med J 10. 133-196 -Roberts AM, Smith KL, Dowell BL, Hubbard AK ( 197ij) Cultural, morphological, cell membrane enzymatic, and neoplastic properties of cell lines derived from a Hodgkin's disease lymph node Cancer Res 38.3033-3043 -Rosenberg SA, Kaplan HS ( 1966) Evidence for an orderly progression in the spread of Hodgkin's disease Cancer Rcs 26. 1225- 123 1 -Rouvierc H ( 1932) Anatomie des lymphatilJues de I'hommc Masson, Paris -Rubin p, Keys H, Mayer E, Antcmann R ( 1974) Nodal recurrcnces following radical radiation therapy in Hodgkin's discasc, AJR 120 536~548 -Ruhl H, Vogt W, Borchert G, Schmidt S, Moelle R, Schaoua H ( 1975) Mixed lymphocyte culture stimulatory and responding capacity of lymphocytes from paticnts with Iymphoproliferative diseascs. Clin Exp ImmunoI19.55-65 Schaadt M, Fonatsch C, Kirchner H, Oiehl V( 1979) Establishment of a malignant, EpsteinBarr virus (EBV)-negativc cell line from the pleura effusion of a patient with Hodgkin's discase, Blut 3ij.185-190 -Schier WW, Roth A, Ostroff G, Schrift MH ( 1956) Hodgkin's disease and immunity Am J Mcd 20'94-99 -Seif GSF, Spriggs AL ( 1967) Chromosome changcs in Hodgkin's disease. J Natl Cancer Inst 39'557-570 Sibbitt WL, Bankhurst AD, Williams RC ( 1978) Studies of cell subpopula tions mcdiating mitogen hyporesponsivene;;s in patients with Hodgkin's disease. 1 Clin Invest 61.55-63 Smithers OW ( 1970) Spread of Hodgkin's disease Lancet 11261-1267- Smithers DW (1973) Modes of spread In; Smithcrs DW (cd) Hodgkin's Disease. Churchill-Livingstonc, Edinburgh London pp 1 07-117- Smithcrs OW, Lillicrap SC, Barnes A (1974) Patterns of lymph node involvement in relation to hypotheses about the modes of spread of Hodgkin's disease Cancer 341779~1786 -Taylor CR (1976) An immunohistological study of follicular lymphoma, reticulum cell sarcoma and Hodgkin's disease. Eur 1 Cancer 1261-75 Twomey 11, l-aughter AH, Farrow S, Douglass CC (1975) Hodgkin's diseasc An immunodepleting and immunosuppressive disorder. 1 Clin Invest 56467~475 -White!aw DM (1969) Chromosome complement of lymph node cells in Hodgkin's disease. Can Med Assoc 1 101. 74-81- Young RC, Corder MP, Haynes HA, De Vita VT ( 1972 ) Oclayed hypersensitivity in Hodgkin's disease A study of 103 untreated patients. Am 1 Med 5263-72Zicgler1B,HansenP,PcnnyR(1975) Intrinsic lymphocyte defect in Hodgkin's disease . analysis of the phytohemagglutinin dose-response. Celllmmunollmmunopathol3:451-460 |