Maximov's Ideas and Modern Models |
|
The Gameleya Institute for Epidemiology and Morphology, Academy of Vet. Med Sciences of USSR. Immunomorphol. Lab., Gameleya Street 18. Moscow D 98, USSR The idea of stromal-hematopoietic cell interactions was the essential
part of Alexander Maximov's theory ofhematopoiesis, which he proposed
more than 60 years ago. According to Maximov (see Figs. 1-4), committed
hematopoietic precursors descend from the hematopoietic stem cells
due to local impacts generated by marrow stroma; this creates the
conditions for hematopoietic cell differentiation [1]. Maximov's
theory was far ahead of his time, and, though Maximov was highly
respected in the scientific community, his concept of local ""differentiation
conditions" operative in hematopoiesis was met with particular skepticism.
Today, Maximov's idea raises no doubt; in fact, it constitutes the
essence of the problem of hematopoietic microenvironment (HME).
What provokes discussions in modern hematology is the exact types
of stromal cells responsible for HME and the mechanisms of stromal-hematopoietic
cell interactions. Maximov assumed that the stromal cells in question
were stromal fibroblasts (reticular cells), but for a long time
many experimental hematologists denied this. Only recently has it
been possible to apply two experimental models for checking the
microenvironmental functions of marrow fibroblasts. The first model
is the transfer of HME by heterotopic transplantation of marrow
cells; the sccond is the establishment of HME in vitro by stromal
cell underlayers in Dexter cultures. The Gameleya Institute for
Epidcmiology and Morphology, Academy of Vet. Med Sciences of USSR.
Immunomorphol. Lab., Gamclcya Street 18. Moscow D 98, USSR Heterotopic
transplantation of marrow cells results in the formation of marrow
organs covered by a bone capsule [2- 5] .Their hem a topoietic cells
are of the recipient origin [6], indicating that engraftment of
some category of marrow cells results in the formation of bone and
an HME suitable for population by hematopoietic cells and for their
proliferation and differentiation. Heterotopic marrow can be retransplanted
repeatedly with similar results, provided the recipients are compatible
with H-2 antigens of the initial donor, not of the intermediate
recipients [7 -8]. This means that HME is transferred by engraftment
of the marrow cells which remain unreplaced by the recipient cells.
Chromosome typing of clonogenic stromal fibroblasts (CFUf) of the
heterotopic marrow confirmed their donor origin [9, 10], and the
problem was to check whether stromal fibroblasts were able to transfer
HME when grafted heterotopically. The in vitro descendents of CFUf
after several passages compose diploid fibroblast culturcs [1113].
Tested by heterotopic transplantation, thcy wcre found to form bone
marrow organs, while engraftment of cultured spleen fibroblasts
(the descendents of spleen CFUf) produced lymphoid organs [14, 15].
Thus, cultured marrow fibroblasts appear to be able to transfer
bone marrow HME. Depending on the origin of marrow fibroblast cultures
(the source CFUfbeing from red or yellow marrow), their engraftment
transferred not only the general pattern of HME, but also such details
as the density of hematopoietic cells in a would-bc marrow [16].
Cultured marrow fibroblasts produce hematopoietic growth factor
(M-CSF, G CFS, GM-CFS, BFUf- and mixed-colony-CSF) which can be
detected in the culture medium [17- 20]. They regulate proliferation
and differentiation of GMCFU: their stimulatory effects were noted
when the target marrow contained few spontaneous colonies, the inhibitory
effects when large numbers of spontaneous GM-CFU were present [21].
Hematopoietic growth factors are also produced by cloned lines of
marrow fibroblasts [22]. However, the direct proof of in vitro microenvironmental
competence of marrow fibroblasts was their ability to establish
HME in Dexter-type cultures. It has been shown [23] that when used
as underlayers, the passaged murine marrow fibroblasts, free from
macro phages and endothelial cells, supported hematopoiesis if seeded
with stromal cell-depleted marrow suspensions. Thus, cultured marrow
fibroblasts transfer HM E, release hematopoietic growth factors
in vitro, and are capable of presenting them in a proper way to
support hematopoiesis in cultures. This confirms Maximov's hypothesis
of the role of marrow fibroblasts in hematopolesIS. The population
of marrow fibroblasts is probably a heterogeneous one, and there
is no evidence that marrow fibroblasts which produce or present
hematopoietic growth factors are the same cells which transfer HME,
and vice versa. It may well be that there are several subpopulations
of marrow fibroblasts with different microenvironmental functions.
At present, fibroblasts including those from nonhematopoietic and
hematopoietic organs look much alike, reminiscent of the situation
with lymphocytes in Maximov's time. The main and most conclusive
sine of fibroblasts (mechanocytes) is interstitial collagen types
I and III synthesis, and few markers of their phenotype and genetic
diversity have been so far ascertained. The diversity does exist,
for instance, between marrow as compared with spleen fibroblasts,
which is proved by the results of their heterotopic transplantation.
The next question regarding HME seems to be the diversity of marrow
fibroblasts including their clonogenic precursor cells. In primary
cultures of marrow cell suspensions the CFUf (CFCf) form adherent-cell
colonies which are cell clones [24, 25]. The colonies are composed
of fibroblasts which synthesize type-1 and -Ill collagen and fibronectin
and lack macrophage markers and VIll-factor-associated antigen [26-30].
Morphologically, the colonies are distinctly heterogeneous within
each culture. Some are composed of elongated or blanket-like fibroblasts
or of a mixture of both; the colonies may include fat cells or have
a mineralized intercellular matrix [39]. These differences can hardly
be regarded as markers of CFCf, the diversity not beeing stable
at passaging and recloning. In situ CFCf are outside the cycle arrested
in Go [31]. Marrow fibroblasts possess PDG F receptors [32] and
in medium with platelet-poor plasma their proliferation and the
CFUf colony formation requires PDGF [33, 34]. It is believed that
serum growth factors, which include PDGF, are sufficient for recruitment
of CFCf into the cycle and that CFUf colony formation in serum-supplemented
medium does not require additional growth stimulation. Yet this
is probably not the case. The efficiency of CFUf colony formation
(CFEf) drops close to zero in lowdensity marrow cultures if they
are depleted of nonadherent cells: 85% of CFCf do not proliferate
at all or pass through one to three cell doublings (Fig. 1). On
the other hand, the CFEf increases dramatically when such adherent
marrow cell cultures are supplemented with irradiated marrow feeder
cells or with platelets. This colony-stimulating activity is not
replaced by additional PDGF and is expressed only in the serum-rich
medium. Being stimulated by platelets each fibroblast precursor
present in marrow cell suspensions turns out to be a clonogenic
stromal cell (Fig.l). Thus, nonstromal marrow cells which accompany
CFCf in marrow cultures 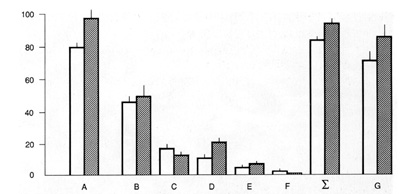
of HME transfer with bone formation, which applies to heterotopic
transplantation of both freshly isolated marrow and single-CFUf-derived
fibroblast colonies. In the heterotopic marrow the CFUf are of donor
origin [9, 10], and it is reasonable to assume that the same applies
to the microenvironmentally competent reticular cells. However,
the ability of fibroblasts from single CFUf-colonyderived heterotopic
bone marrow organs to support hematopoiesis in vitro, and their
donor origin (which would be the proof of the above speculation)
was not tested up to now. Anyway, the hierarchy of marrow precursors
awaits further studies. As far as Maximow's contribution to the
problems of HME is concerned, it is impossible to omit his last
work, entitled "Cultures of blood leukocytes. From lymphocyte and
monocyte to connective tissue." [40]. It describes the formation
of fibroblasts in plasma-clot cultures of guinea-pig blood cells.
Subsequently, his results were put in question on the grounds of
two possible objections, namely that the source of fibroblasts might
be fragments of vessel walls which contaminate the blood during
sampling, and that the cells in question were not fibroblasts (for
references, see [41]). The first objection proved to be invalid
when a CFUf colony assay was carried out 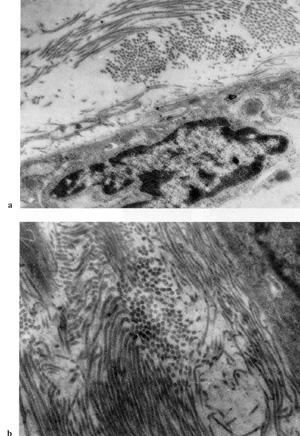
with blood cells. It turned out that the incidence of CFU f colonies
in guinea-pig and rabbit leukocyte cultures did not change with
the number of punctures performed for blood sampling [42]. It has
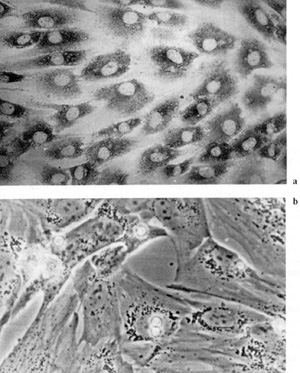
also been shown that fibroblasts in blood-derived CFUf colonies
synthesize collagen type I [43] and lack VIII-factorassociated antigen
and macrophage determinant Mac I [44], which confirms their fibroblast
nature (Fig. 2, 3). It remains unknown from where CFUf migrate into
blood, where they settle (if they do), and why blood-derived CFUf
are not detectable in some mammals, including human beings. The
presence of fibroblast precursors in blood discovered by Maximov
is related to many unsolved problems of HME, in particular, to the
possibility of CFUf repopulation; CFUf circulation in blood does
not prove it at all. The story of the circulating fibroblast precursor
cells demonstrates once again that not only Maximov's ideas, but
also his experimental results are so topical that Professor Alexander
Maximov almost remains a participant of presentday research (Fig.
5). 
 Fig.5a. Preparation of plasma for plasma-clot cultures  Fig. 5b. Placing tissue fragments in culture medium  Fig.5c. Kaissug hangrug-drop cultures
in
1. Maximov AA (1906) Über experimentelle Erzeugung von Knochenmarks-Gewebe.
Anat Anz 28.24-38 |