to Study the Organization of the Macrophage System |
In: Zander AR et al. (eds) Gene Technolgy, Stem Cell and Leukemia Research, Nato ASI Series H: Cell Biology, Vol 94, Springer-Verlag, Berlin Heidelberg New York London |
|
Department of Immunology Central Clinical Hospital Military School of Medicine PLOO-909 Warsaw Poland Physiological role of many molecules and cells can be investigated by evaluating animals possessing genetically determined alterations in the production of those molecules and/or cells. While animals with either knockouts of genes for various molecules or transgenic for these molecules could be at present experimentally created, there is still not fully explored potential of natural mutants, the so called "experiments of nature" (Good, 1991 ). In particular, there are several natural mutants with osteopetrosis: a disorder of osteoclasts i.e. cells related to macrophages, and they all may have alterations in other parts of the macrophage system (Wiktor-Jedrzejczak et al., 1981, Marks 1987). One of such models is the osteopetrotic op/op mouse (Marks and Lane, 1976) . This mutant was found to possess very severe deficiency of macrophages, secondary to deficiency of a growth factor (Wiktor-Jedrzejczak et al.,1982). In particular, it allowed the identification of the total absence of a major macrophage growth factor: colony stimulating factor 1 (CSF-1 or M-CSF; Wiktor-Jedrzejczak et al., 1990, Felix et al., 1990) as the cause of cellular deficiences. In turn, CSF-1 absence was found to be due to inactivating mutation of the gene for that factor (Yoshida et al., 1990). In consequence of these studies, the first mutant with severe congenital deficiency of macrophages became available for the investigation of the organization of the macrophage system, the function and diversity of cells belonging to that system and for verification of the evidence regarding this system obtained using other models (VanFurth et al., 1972, Nathan and Cohn, 1985, Gordon, 1986). Simultaneously, this model became molecularly defined. Macrophage lineage belongs to myelopoiesis but differs from other lines of myeloid differentiation in that: -cells leaving the bone marrow, i.e. monocytes are not yet fully functionally mature, and in some species including mice they may even proliferate; -there is a striking functional and phenotypic diversity among the end cells of the system, which include various forms of tissue macrophages as well as cells that are not phagocytes, such as dendritic cells and osteoclasts (Auger and Ross, 1992); -there is an additional very large potential to increase this diversity of mature cells by their activation, with dramatic enhancement of some functions already expressed by resting cells, and the appearance of many new functions (Adams and Hamilton, 1992) . Classically, macrophage differentiation in the bone marrow was considered to begin at the level of hematopoietic stem cell, and then to proceed through the stage of bipotent neutrophil-macrophage progenitor to the first identified cell of the lineage namely the monoblast, and subsequently to monocytes, and macrophages, as well as to the other end cell of the system (VanFurth, 1993) .However, in addition to these studies, also very primitive macrophage progenitor cells have been identified by Bradley and Hodgson ( 1979) suggesting that some macro phages may derive from progenitors unique to their line of differentiation and descending directly from the stem cells. Subsequent studies have suggested even greater heterogeneity of macrophage progenitors (Bertoncello et al., 1991, Suda et al., 1983). There are three identified growth factors, that when individually added in vitro to macrophage progenitors stimulate their growth and maturation to macrophages (Prystowsky et al., 1984, Koike et al., 1986, Falk and Vogel, 1988, Wiffeils et al., 1993). Such primary macrophage growth factors (Metcalf, 1991) include CSF-1, granulocyte-macrophage (GM)-CSF, and interleukin 3 (IL-3). CSF-1 is a large dimeric cytokine with homology to Steel Factor, and binding to a dimeric receptor of immunoglobulin superfamily with intrinsic tyrosine kinase activity: c-fms. Both GM-CSF and IL-3 belong to hematopoietin family and their receptors belong to the family of hematopoietin receptors. The gene for CSF-1 is located on chromosome 3 at the op locus (Gisselbrecht et al.,1989), is composed of 10 exons, and is producing five different mRNA by alternative splicing (reviewed by Stanley, 1994) .There are at least three different (N-terminus identical) protein forms of CSF-1 : -soluble proteoglycan with largest protein part of 522 aminoacids; -soluble glycoprotein of 406 aminoacids, and: -membrane-spanning glycoprotein of 224 aminoacids, which may be shed and also contribute to soluble CSF-1 . Genes for both GM-CSF and IL-3 are located on chromosome 11 and code for only one protein form of each factor (Gasson, 1991, I hie and Weinstein, 1986). Receptors for GM-CSF and IL-3 have common beta subunit (responsible for signal transduction to the cell inside) and unique alpha subunits (responsible for cytokine binding). In the mouse there is an additional beta subunit exclusive for IL-3 receptor (Miyajima et al.,1992). While IL-3 is only a paracrine factor acting at the vicinity of cells that produce it, GM-CSF is present in the circulation in small amounts (Cheers et al., 1988), suggesting that it may participate in steady-state regulation. However, the most of circulating macrophage colony stimulating activity is due to CSF-1, which is both endocrine (i.e. normally present in peripheral blood), paracrine, and cell contact molecule. Moreover, while both GM-CSF and IL-3 are considered to be mainly induced factors (Gasson, 1991, I hie and Weinstein, 1986), CSF-1 has considerable level of constitutive expression, and therefore, may playa major role in steady-state regulation as opposed to stress regulation, which may be a predominant role for GM-CSF and IL-3 as well as for other molecules. However, there is a considerable overlap between functions of CSF-1 , GM-CSF and IL-3, and it is extremely difficult to dissect the actual role of each molecule in the regulation of the system using normal mouse models.
The op/op mouse is deprived of all forms of CSF-1 (Wiktor-Jedrzejczak et al., 1990) as a consequence of the insertion of thymidine in position 262 of CSF-1 gene (Yoshida et al., 1990). This insertion shifts the reading frame and produces stop codon 21 bases downstream. Such defect should lead to the production of truncated protein, shorter than any known bioactive form of CSF-1 (Heard et al., 1987). Although theoretically possible, there is no evidence for the repair of this lesion in mutant mice, and the described partial resolution of osteopetrosis in old op/op mouse (Marks and Lane, 1976, Begg et al., 1993) is considered to be the effect of other compensatory mechanisms rather than being due to the leak in the defect (Wiktor-Jedrzejczak, 1993b). Morever, the utilization of the op/op mouse model is facilitated by the availability of human recombinant CSF-1 (active in murine system) , that can be used in reconstitution experiments (Ladner et al., 1987, Halenbeck et al., 1989). Consequently, the role of CSF-1 in the regulation of the macrophage system can be studied by a combination of the analysis of deficiences of cells of this system in mutant mice with analysis of mutant animals having reconstituted circulating level of CSF-1 by the administration of recombinant form of the factor .
The macrophage deficiency in the op/op mouse is severe but not
absolute. Functionally competent macrophages are present (although
in reduced number) in mutant animals (Wiktor-Jedrzejczak et al.,
1992b) .Moreover, very profoud differences exist in the degree of
affection of various local macrophage populations. This heterogeneity
concerns not only different organs but also different specific locations
within the same organ e.g. spleen. There are tissues, where the
number of resident macrophages is reduced to less than 5% of the
normal and sometimes it is even negligible. They include peritoneal
cavity (where the defect was originally identified: Wiktor-Jedrzejczak
et al., 1982) pleural cavity, muscle, skin, periostium, synovium,
uterus, kidney and peripheral blood monocytes (Naito et al., 1991,
Wiktor-Jedrzejczaket al., 1992b, Witmer-Pack et al., 1993, Cecchini
et al., 1994) as well as spleen metallophils (Witmer-Pack et al.,1993,
Cecchini et al.,1994) and osteoclasts (Marks and Lane, 1976, Marks,
1982). Organs, where the deficiency is less pronounced, and the
number of macrophages is between 10 and 80% of the normal include
the liver, other than metallophils populations of spleen macrophages,
lung, intestine, salivary glands, adrenals, bladder as well as brain
microglial cells (Naito et al., 1991, WiktorJedrzejczak et al.,1992b,
Witmer-Pack et al.,1993, Cecchini et al., 1994). On the other hand,
epidermal Langerhans cells, dendritic cells of lymphoid organs,
as well as macrophages present in these organs are quantitatively
normal in the op/op mice (Takahashi et al., 1992, Witmer-Pack et
al., 1993, Takahashi et al., 1993, Cecchini et al.,1994), what suggests
that they are completely CSF-1 independent. Restoration of circulating
CSF-1 by systemic administration of recombinant form of the factor
corrected only some near- completely depleted macrophage-related
populations including osteoclasts, monocytes, kidney macrophages,
and spleen metallophils, as well as some partially depleted populations
including bone marrow, spleen and liver macrophages (Wiktor-Jedrzejczak
et al., 1991, Kodama et al., 1991, Cecchini et al., 1994). Such
populations as peritoneal cavity macrophages could only be restored
by local CSF-1 administration, and it was hypothetized that this
is due to the existence of blood-tissue barrier for circulating
CSF-1 and to the exclusively local control of macrophage populations
in many tissues (WiktorJedrzejczak et al., 1991 ). This hypothetical
barrier was later found to be operative for almost all other local
macrophage populations (Cecchini et al., 1994) in addition to the
peritoneal and pleural cavities. On the other hand, these observations
may also be explained by the requirement for increasing CSF-1 gradient
for monocyte migration to tissues. According to this concept, monocytes
could only migrate from locations with lower CSF-1 concentration
(blood) to higher CSF-1 concentration (tissues) . Whatever the answer,
the reported combined data suggest that the local macrophage populations
could be divided into: -completely CSF-1 dependent; -partially CSF-1
dependent, and: -CSF-1 independent. The first two subpopulations
could be further subdivided into: -dependent on systemic CSF-1 ,
and; -dependent on local CSF-1. Moreover, there appears to be a
certain logic in distinction between organs with considerable CSF-1
independent resident macrophage population, and organs without such
population. The organs with considerable CSF-1 independent macrophage
population are mainly those that possess large total macrophage
populations such as the liver, spleen, lungs, intestine, and that
are at high risk of exposure to microorganisms, and particulate
materials such as cell debris and other. Almost all other organs
appear to have only CSF-1 dependent resident macrophage population,
and they are generally at low risk of exposure to stimuli that require
macrophage action. However, also in these latter organs in the op/op
mouse, it is easy to elicit macrophages at the absence of CSF-1
by for instance injection of endotoxin (Wiktor-Jedrzejczak et al.,
unpublished observations). Furthermore, organs with normally large
resident macrophage population such as the liver and spleen have
their CSF-1 dependent macrophages under control of circulating,
and not only of locally produced CSF-1 .Therefore, it appears that
the presence of resident macrophage population is these organs is
assurred in multiple ways, that is by circulating CSF-1 , local
CSF-1 , and by other primary macrophage growth factors. In contrast,
in most other organs resident macrophages are almost exclusively
dependent on local CSF-1 (Fig. 1) .However, there are also organs
such as the kidneys or specific subpopulations such as spleen metallophils,
which are almost exclusively CSF-1 dependent, and which are dependent
on circulating CSF1 (Cecchini et al., 1994). Additionally, it has
to be pointed out that deficient macrophage populations in the op/op
liver, spleen and other organs are maintained at the near absence
of monocytes. Although diminished, these populations still constitute
about 50% of the normal macrophage populations in these organs and
are, therefore, of considerable size. This may suggest, that either
only CSF-1 dependent resident macrophage populations require constant
supply of monocytes or CSF-1 independent macrophages are replenished
by a tiny monocyte subpopulation with very high turnover . 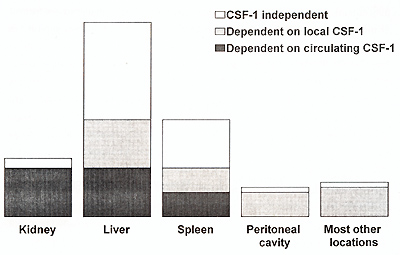
Hematopoietic organs of the op/op mice possess large numbers of
macrophage progenitors, with their frequency being normal in the
bone marrow and increased in the spleen (Wiktor-Jedrzejczak et al.,
1992b). Only after calculation of the total mouse macrophage progenitor
number it was possible to detect a deficiency in this parameter
in the op/op mouse and this deficiency could be a secondary consequence
of reduced marrow due to osteopetrosis rather than specifically
related to CSF-1 absence. This suggests that CSF-1 is not necessary
for the generation of macrophage progenitors from the hematopoietic
stem cells. Macrophages could be generated in vivo in the op/op
mice after administration of CSF-1 within only 48 hours (Wiktor-Jedrzejczak
et al., in preparation for publication). This suggests that even
quite late macrophage progenitors are formed at the absence of CSF-1
, and that only then they reach block preventing further maturation.
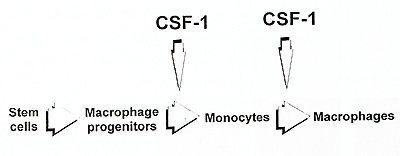
Consequently, only macrophage formation and not macrophage progenitor formation appears to be considerably CSF-1 dependent (Fig. 2). The process of macrophage formation may be further divided into formation of monocytes from macrophage progenitors and formation of macrophages from monocytes. Absence of more than 95% of monocytes in the op/op mouse and their restoration after CSF-1 treatment (Kodama et al.,1991) suggests that this process is near completely CSF-1 dependent. This agrees to some extent with published models of the development of macrophage system, where CSF-1 and other macrophage factors have been suggested to act mainly in the bone marrow effecting the production of monocytes (VanFurth, 1993, Johnson, 1993}. However, the very presence of profound macrophage deficiencies in the op/op mice suggests that the role of CSF-1 in transition of monocytes to the tissues, and in their further maturation to macrophages resident there, is much more important. Clearly, this suggests that the essential CSF-1 role in macrophage maturation is extramedullary, and that CSF-1 is not necessary in the bone marrow, but for most of the tissues (listed earlier) it is necessary in those tissues to maintain supply of monocytes, formation of macrophages, and local survival of these latter cells. This is supported also by the observation, that the CSF-1 treated op/op mouse has plenty of monocytes and still possess profound local macrophage deficiencies (Wiktor-Jedrzejczak et al., 1991, Cecchini et al., 1994). The data from other models suggest that CSF-1 is not necessary for macrophage activation (Evans, 1991) .Our studies suggest that it is clearly not necessary for the in vivo activation of those CSF-1 independent macrophages that are still present in the op/op mouse (Wiktor-Jedrzejczak et al., unpublished observations). However, the dependency of activation of CSF-1 dependent macrophages on CSF1 has not been experimentally approached in the mutant mice. This is testable by restoring CSF-1 dependent macrophages in the op/op mouse with exogenous CSF1, withdrawing this factor and testing macrophage activation at CSF-1 absence. Therefore, the results of such experiment should provide more definite answer to this Question.
In addition to the op/op mouse, animals with knockouts of a gene for the 2nd major primary macrophage factor: GM-CSF have been created recently (Dranoff et al., 1994, E. Stanley et al.,1994). Moreover, also a double knockout: GM-CSFknockout-op/op mice have been bred and partly characterized (lieschke et al., 1994). These models complement very well the advantages of the op/op mouse. Similarly to the op/op mouse, these mutants possess large numbers of macrophage progenitors (E. Stanley et al.,1994, lieschke et al., 1994), confirming that CSF-1 is not necessary for their formation, and suggesting that also GM-CSF is playing only a minor role in that process. In agreement with a notion that most of monocytes are produced under CSF-1 influence, GM-CSF knockout mouse possess normal number of these cells (Dranoff et al., 1994, E. Stanley et al., 1994). However, also double knockout: GM-CSF knockout-op/op mouse still possess some monocytes (lieschke et al., 1994), suggesting that some other factor at the absence of both GM-CSF and CSF-1 is capable of maintaining formation of a few monocytes, and that CSF-1 independent macrophages present in the op/op mouse are not exclusively GM-CSF dependent macrophages. One possible candidate molecule for the supporting the production of CSF-1 independent macrophages is IL-3. However, the fact that it is produced only by activated T cells (Ihie and Weinstein, 1986) may suggest that also some other molecules may playa role in that process. The discussion of contribution of other than CSF-1 factors to macrophage presence in the tissues has to begin from the appreciation that considerable numbers of resident macrophages are present in the op/op mice in such organs as liver and spleen suggesting that they can reach full functional maturity without CSF-1. This may suggest, that in agreement with the model proposed earlier (Wiktor-Jedrzejczak, 1993b) other than CSF-1 primary macrophage growth factors also playa role in terminal maturation of macrophages, and contribute to the formation and maintenance of resident (as opposed to induced) cells. Both published reports concerning GM-CSF knockout mice failed to identify numerical macrophage deficiencies and pointed out pulmonary proteinosis as the major abnormality present in these mice. Whether this proteinosis due to surfactant accumulation is secondary to the deficiency of a few alveolar macrophages dependent on GM-CSF is still an open question. Theoretically, it is possible that GM-CSF may control surfactant production also by a macrophage-independent mechanism. However, the analysis of macrophage deficiencies in these mice was not sufficiently detailed to exclude the presence of even quite significant local deficits. Until such data are available, it is difficult to answer such basic question as: whether CSF-1 dependent and GM-CSF dependent macrophage subpopulations in organs such as liver are regulated independently of each other or whether the total liver macrophage population is maintained by the combined activity of all macrophage growth factors? However, the regular character of macrophage deficiencies in the op/op mouse may suggest that they are created by subtraction of an inherently regulated CSF-1-only- dependent subpopulation. In fact, data are available from other model that CSF-1 level and CSF-1 dependent macrophage subpopulation that is consuming CSF-1 form an integral regulatory feedback system (Bartocci et al., 1987). Besides, the op/op mouse under stress conditions such as bacterial infection can produce large numbers of macrophages. For instance in the course of E. coli fecal peritonitis the mutant mice are capable of eliciting millions of macrophages in peritoneal cavity within 48 hours postinfection (Wiktor-Jedrzejczak et al., submitted for publication). This suggests that CSF-1 independent (i.e. dependent on other macrophage growth factors) formation of induced macrophages is efficient in the op/op mouse, in contrast to deficient formation of resident macrophages. Interestingly, the induced local expansion of macrophages may originate from local progenitors. This is supported by a recent observation that in the presence of inflammatory stimulus such as glucan, local (liver) macrophages can considerably expand, at the absence of CSF-1 , and at the near absence of monocytes (Takahashi et al., 1994).
We have suggested earlier, that CSF-1 dependent macrophages (absent
in the op/op mouse) play mainly regulatory role through release
of cytokines, while CSF-1 independent macrophages (present in the
op/op mouse) are responsible primarily for the immune functions
of macrophages (Wiktor-Jedrzejczak et al. , 1992a) .It has to be
understood that in order to be called macrophages, cells have to
possess common set of functions including phagocytosis. Other studies
comparing CSF-1 induced and GM-CSF induced macrophages in vitro
have shown that GM-CSF induced macrophages expressed higher levels
of class II MHC (Doherty et al., 1993) what is in agreement with
suggestion on primarily immune function of these cells. However,
the same authors have also suggested that GM-CSF induced macrophages
are better producers of cytokines including IL-1 , IL-6 and TNF-alfa
than CSF-1 induced macrophages. This is in contrast to the postulated
major role of these latter cells in that process. On the other hand,
not only efficiency of production on the per cell basis counts in
vivo, but also availability of cells. From that point of view, in
most locations the vast majority of resident macrophages are dependent
on CSF-1 and they have to provide the respective cytokines during
early phases of tissues response to trauma or infection (Fig.3).
This notion is supported by the presence of TNF-alfa, and IL-1 alfa
deficiencies in the op/op mouse (WiktorJedrzejczak et al., 1992a,
Szperl et al., 1995). 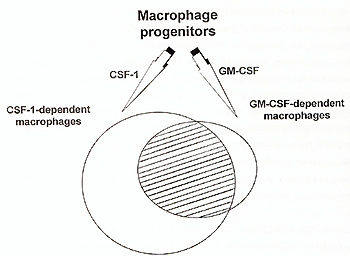
At present, there appears to be no data supporting the existence of separate CSF-1 dependent and CSF-1 independent macrophage progenitors. All macrophage progenitors seem to be able to respond to both CSF-1 and GM-CSF (Metcalf and Nicola, 1992). There is no synergy between these factors, what may suggest that they act on the same cell population. However, after macrophages are formed, in addition to functions shared by macrophages formed under the influence of any factor, there appear to be functions that are exclusively dependent on CSF-1 or GM-CSF. Only CSF-1 dependent resident peritoneal macrophages are capable of recruiting lymphocytes to peritoneal cavity (Kalinski et al.,1993). On the other hand, eicosanoids production has been recently shown to be an exclusive function of GM-CSF and IL-3 produced macrophages, and not of CSF-1 induced macrophages (Shibata et al.,1994).
The studies employing the op/op mouse challenge several established views concerning the organization of the macrophage system and provide novel insights into regulation of macrophages at the tissue level. The model is far from being explored and awaites application to the studies reappraising various roles classically assigned to macrophages. Moreover, its possibilities may be increased by breeding mice combining CSF-1 deficiency with deficiencies of other earlier or later acting macrophage growth factors and macrophage activators such as interferon-gamma. An example of this approach: breeding the op/op mouse possessing knockout of GMCSF gene is already available (Lieschke et al., 1994) .It is a strong belief of the present author that these models would allow final dissection of the organization and function of the macrophage system.
This study was supported in part by grants 4131791 01 and 44381
91 02 from
Adams DO, Hamilton T A ( 1992) Molecular basis of macrophage activation : diversity and its origins. In: Lewis CE, McGee JOD (eds) The Natural Immune System: The Macrophage. IRL Press, Oxford, pp.75-114 Auger MJ, Ross JA (1992) The biology of the macrophage. In: Lewis CE, McGee JOD (eds) The Natural Immune System: The Macrophage. IRL Press, Oxford, pp.2-74 Bartocci A, Mastrogiannis DS, Migliorati G, Stockert AJ, Wolkoff AW, Stanley EA ( 1987) Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci USA 84:6179-6183 Begg SK, Aadley JM, Pollard JW, Chisholm OT, Stanley EA, Bertoncello I (1993) Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med 177:237-242 Bertoncello I, Bradley TR, Hodgson GS, Dumlop JM (1991) The resolution, enrichment, and organization of normal bone marrow high proliferative potential colony-forming cell subsets on the basis of rhodamine-123 fluorescence. Exp Hematol 19: 174-178 Bradley TR, Hodgson GS ( 1979) Detection of primitive macrophage progenitor cells in bone marrow. Blood 54:1446-1450 Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm 0, Hofstetter W, Pollard JW, Stanley ER (1994) Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120: 1357-1372 Cheers C, Haigh AM, Kelso A, Metcalf D, Stanley ER, Young AM ( 1988) Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun 56:247-251 Doherty TM, Kastelein R, Menon S, Andrade S, Coffman RL (1993) Modulation of murine macrophage function by IL-13. J Immunol 151 :7151- 7160 Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC (1994) Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264:713-716 Evans R ( 1991) Clarification of the potential role of CSF-1 in activation of macrophages. J Leukocyte Bioi 50:316 Falk LA, Vogel SN ( 1988) Comparison of bone marrow progenitors responsive to granulocyte-macrophage colony stimulating factor and macrophage colony stimulating factor-1. J Leukocyte Bioi 43:148-157 Felix R, Cecchini MG, Hofstetter W, Elford PR, Stutzer A, Fleisch H (1990) Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Mineral Res 5:781-789 Gasson JC (1991) Molecular physiology of granulocyte-macrophage colony stimulating factor. Blood 77:1131-1145 Gisselbrecht S, Sola B, Fichelson S, Bordereaux D, Tambourin P, Mattei M-G, Simon D, Guenet J-L (1989) The murine M-CSF gene is localized on chromosome 3. Blood 73: 1742-1746 Good RA ( 1991) Experiments of nature in the development of modern immunology. Immunol Today 12:283-286 Gordon S (1986) Biology of the macrophage. J Cell Sci Suppl 4:267-286 Halenbeck R, Kawasaki E, Wrin J, Koths K ( 1989) Renaturation and purification of biologically active recombinat human macrophage colony-stimulating factor expressed in E. coli. Bio/Technology 7:710- 715 Heard JM, Roussel MF, Rettenmier CW, Sherr CJ ( 1987) Synthesis, posttranslational processing, and autocrine transforming activity of a carboxylterminal truncated form of colony stimulating factor-1. Oncogene Res 1 :423-440 Ihle JN, Weinstein Y (1986) Immunological regulation of hematopoietic/lymphoid stem cell differentiation by interleukin 3. Adv Immunol 39: 1-49 Johnson RB Jr ( 1993) Monocytes and macrophages. In lachmann PJ, Peters K, Rosen FS, Walport MJ (eds) Clinical Aspects of Immunology. Blackwell, Boston, Voi.1 ,pp.467-479 Kalinski P, Urbanowska E, Kawiak J, Hoser G, Aukerman Sl, Wiktor-Jedrzejczak W ( 1993) CSF-1 dependent but not GM-CSF dependent macrophages recruit lymphocytes to peritoneal cavity. Exp Hematol 21 : 1014 Kodama H, Yamasaki A, Nose M, Nijda S, Ohgame Y, Abe M, Kumegawa M, Suda T (1991) Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med 173:269-272 Koike K, Stanley ER, Ihie JN, Ogawa M (1986) Macrophage colony formation supported by purified CSF-1 and/or interleukin 3 in serum-free culture: evidence for hierarchical difference in macrophage colony-forming cells. Blood 67:859-864 Ladner MB, Martin GA, Noble JA, Nikoloff DM, Tal R, Kawasaki ES, White T J (1987) Human CSF-1 : gene structure and alternative splicing of mRNA precursors. EMBO J 6:2693-2698 Lieschke GJ, Stanley E, Grail D, Hodgson G, Sinickas V, Gall JAM, Sinclair RA, Dunn RA ( 1994) Mice lacking both macrophage- and granulocyte-macrophage colony stimulating factor have macrophages and coexistent osteopetrosis and severe lung disease. Blood 84:27-35 Marks SC Jr ( 1982) Morphological evidence of reduced bone resorption in osteopetrotic (op) mice. Am J Anat 163:157-167 Marks SC Jr (1987) Osteopetrosis: multiple pathways for the interception of osteoclast function. Appl Pathol 5: 172-183 Marks SC Jr, Lane PW ( 1976) Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered 67: 11-18 Metcalf D ( 1991) Control of granulocytes and macrophages: molecular, cellular , and clinical aspects. Science 254:529-533 Metcalf D, Nicola NA (1992) The clonal proliferation of normal mouse hematopoietic cells: enhancement and suppression by colony-stimulating factor combinations. Blood 79:2861-2866 Miyajima A, Kitamura T, Harada N, Yokota T, Arai K-j (1992) Cytokine receptors and signal transduction. Annu Rev Immunol 10:295-331 Naito M, Hayashi S-I, Yoshida H, Nishikawa S-I, Shultz LD, Takahashi K (1991 ) Abnormal differentiation of tissue macrophage populations in' osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol 139:657-667 Nathan C, Cohn ZA (1985) Cellular components of inflammation: monocytes and macrophages. In: Kelley WN, Harris ED Jr, Ruddy S, Sledge CB (eds) Textbook of Rheumatology. 2nd Ed. WB Saunders, Philadelphia, pp.144-169 Prystowsky IB, Otten G, Naujokas MF, Vardiman J, I hie JN, Goldwasser E, Fitch FW ( 1984) Multiple hemopoietic lineages are found after stimulation of mouse bone marrow precursor cells with interleukin 3. Am J PathoI117:171-179 Shibata Y, Bjorkman DR, Schidt M, Oghiso Y, Volkman A (1994) Macrophage colony-stimulating factor-induced bone marrow macrophages do not synthesize or release prostaglandin E2. Blood 83:3316-3323 Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JAM, Maher DW, Cebon J, Sinickas V, Dunn AR ( 1994) Granulocyte/macrophage colonystimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 91:5592-5596 Stanley ER ( 1994) Colony stimulating factor-1 (macrophage colony stimulating factor). In The Cytokine Handbook, Academic Press, New York, pp.387-418 Suda T, 5uda J, Ogawa M ( 1983) 5ingle-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad 5ci U5A 80:6689-6693 Szperl M, Urbanowska E, Ansari AA, Wiktor-Jedrzejczak W ( 1995) Increased resistance of macrophage-deficient, TNF-alfa-deficient, IL-1 alfa-deficient op/op mouse to endotoxin. Ann NY Acad 5ci (in press) Takahashi K, Naito M, 5hultz LD (1992) Differentiation of epidermal Langerhans cells in macrophage colony-stimulating-factor-deficient mice homozygous for the osteopetrosis (op) mutation. J Invest Dermatol 99:465-475 Takahashi K, Naito M, 5hultz LD, Hayashi 5-1, Nishikawa 5-i ( 1993) Differentiation of dendritic cell populations in macrophage colony-stimulating factor deficient mice homozygous for the osteopetrosis (op) mutation. J Leukocyte Bioi 53: 19-28 Takahashi K, Naito M, Umeda 5, 5hultz LD ( 1994) The role of macrophage colonystimulating factor in hepatic glucan-induced granuloma formation in the osteopetrosis mutant mouse defective in the production of macrophage colonystimulating factor. Am J Pathol 144: 1381-1392 VanFurth R (1993) Cell biology of mononuclear phagocytes. In VanFurth R (ed) Hemopoietic growth factors and mononuclear phagocytes. Karger, Basel, pp.1-9 VanFurth R, Cohn ZA, Hirsch JG, Humphrey JH, 5pector WG, Langevoort HL (1972) The mononuclear system. A new classification of macrophages, monocytes, and their precursor cells. Bull WHO 46:845-852 Wiffeils JFAM, Derover Z, Kraal G, Beelen RHJ (1993) Macrophage phenotype regulation by colony-stimulating factors at bone marrow level. J Leukocyte Bioi 53:249-255 Wiktor-Jedrzejczak W ( 1993a) Cure of osteopetrosis in op/op mice by bone grinding and good food? Exp Hematol 21:1314-1315 Wiktor-Jedrzejczak W ( 1993b) In vivo role of macrophage growth factors as delineated using C5F-1 deficient op/op mouse. Leukemia 7:5117-5121 Wiktor-Jedrzejczak W, Ahmed A, 5zczylik C, 5kelly RR ( 1982) Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med 156: 1516-1527 Wiktor-Jedrzejczak W, Ansari AA, Szperl M, Urbanowska E (1992a) Distinct in vivo functions of two macrophage subpopulations as evidenced by studies using macrophage-deficient op/op mouse. Eur J Immunol 22: 1951-1954 Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER ( 1990) Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 87:4828-4832 Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell KW, Ahmed-Ansari A, Ostertag W (1992b) CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol 20: 1004 1010 Wiktor-Jedrzejczak W, Skelly RR, Ahmed A (1981) Hematopoietic stem cell differentiation and its role in osteopetrosis. In: Gerschwin ME, Merchant B (eds) Immunologic defects in laboratoryanimals. Plenum Press, New York, vol.1 :51-77 Wiktor-Jedrzejczak W, Urbanowska E, Aukerman SL, Pollard JW, Stanley ER, Ralph P, Ansari AA, Sell KW, Szperl M ( 1991) Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp Hematol 19: 1049-1054 Witmer-Pack MD, Hughes DA, Schuker G, Lawson L, McWilliam A, Inaba K, Steinman RM, Gordon S ( 1993) Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Science 104: 1021-1029 Yoshida H, Hayashi S-I, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz D, Nishikawa S-I (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442-444 |