|
Institute or Biogeochemistry and Marine Chemistry at the Center or Marine Research and Climatology, University or Hamburg, Hamburg, FRG.
Aside from helium, living and cosmic matter arc principally composed of the same chemical elements, hydrogen oxygen-carbon-nitrogen, reading in order of abundance, as: C -O -H- N vs H -O- C- N. In contrast, the Earth has an entirely different bulk composition iron-oxygen-silicon-magnesium. In comparing the various patterns one might suggest that living matter sprouts directly out of the ancestral universal matrix, whereas terrestrial matter must be a late derivation product of that matrix. Only oxygen ranks high in all three compartments: cosmos, life, and earth. Thus, it is tempting to look for the roots of life in outer space. Actually, such an attempt is in strong contrast to textbook dogma where the origin of life presupposes a synthesis of vital monomers in a reducing terrestrial atmosphere by means of high energy radiation. A subsequent "raining out" is assumed to have led to a "primordial organic soup" from which physiologically interesting polymers and eventually the first living cell arose (Oparin 1953). To prime the pump of my antithesis, a few details on the distribution of matter in interstellar space and its fractionation in the course of solar and planetary evolution are needed. Its further assemblage in the direction of a workable cellular system by way of mineral templates and globular aggregates will represent the essence of my subsequent presentation.
Our galaxy, the Milky Way, would look if seen from the outside
somewhat like a galaxy in Ursa Major, M81, just 9 million light
years away from us (Fig. 1). About lOO billion stars are contained
in this magnificent spiral about 100000 light years across, and
quite a few may have planets like the Sun. Our solar system would
occupy a place close to the medium plain of the spiral about one
third the distance of the total diameter removed from the galactic
center. We need roughly 250 million Earth years to circumnavigate
the galactic center, Accordingly, this period of time has been defined
as 1 cosmic year. Of the mass we can account for in our galaxy,
about 95% resides in stars and the rest is interstellar gas and
dust in a ratio of about 99 to 1. However, this interstellar matter
is not uniformly distributed throughout the galaxy but is concentrated
in the form of clouds in regions close to the galactic plane of
symmetry, where new stars are born. To most of us, clouds are seen
more in conjunction with weather and climate. Namely, water vapor
will condense and form clouds of different sizes and shapes, which
are freely moving, can become dispersed or aggregate, and form a
cloud cover. Some regions are more cloudy than others. In due course,
they will rain out and the meteorological cycle will commence again
with the evaporation of water bodies. In analogy, interstellar clouds,
a few 
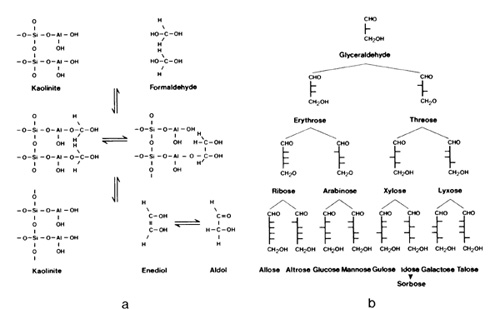
Fig. 4. Accretion disk across solar system from Mercury
to Neptune. Variations in temperature and oxidation states in the
assumed growth regions or meteorites and cometary bodies 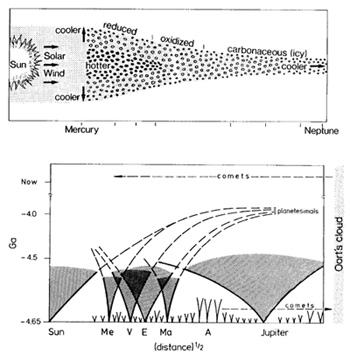
Rock-forming minerals are principally composed of oxygen ions which have as their main coordination partners silicon, aluminum, iron, calcium, magnesium, sodium, and potassium ions. Crust and upper mantle may thus be viewed as an ionic oxysphere. Crystals may contain charge deficiencies, structural irregularities, lattice defects, and, in hydrated varieties such as clays, may even develop hydrogen bonds. To structure our discussion, I will begin with a process commonly described under the heading "epitaxis," a term derived from the Greek tassein, meaning to arrange or to organize. The growth of crystalline material on other crystal surfaces is a well-studied subject in the field of crystallography. Epitaxis can also proceed on organic templates with the resultant formation of biominerals in teeth, bones, or shells. Furthermore, organic polymers can promote the synthesis of other organic polymers, and the living cell is vivid proof of that. Finally, mineral surfaces may provide sites for activation and protection of functional groups displayed by organic molecules, and may accordingly serve as polymerization matrix. Thus, one can distinguish between four systems in which one partner represents the template and the other partner the epitaxial product (Table 1 ). Epitaxis on solid-state surfaces should be viewed in relation to catalysis because both processes follow a similar reaction path. Catalysis represents a process in which a solid-state surface "tries" to establish a thermodynamically favorable phase transition structure with the adsorbent. Phase-transition structures emerge which, when chemically stable, lead to
Template > Product oriented intergrowth. In contrast, should transition structures
introduce a chemical change of the adsorbent such as polymerization,
hydrogenation, dehydrogenation, etc., we are dealing with catalysis.
Principles of cellular catalysis, as, for example, executed by enzymes,
are identical to those observed in mineral systems. Catalysis constitutes
a flow of epitaxial associations, whereas epitaxis involves a "frozen
in" transition structure provided by a morphological catalyst. With
the help of clay minerals, chemical synthesis of a number of physiologically
interesting polymers has been successful; this particularly concerns
the formation of peptides. The mechanism involves carboxyl activation
and the inactivation of functional groups not participating in the
formation of the amide bond by so-called protective groups displayed
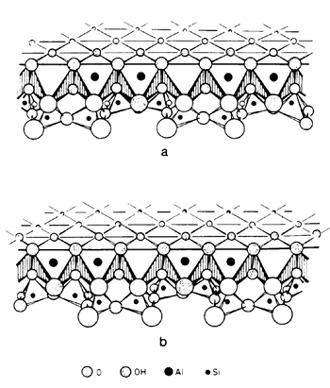
along mineral surfaces. The relationships for a kaolinite-amino
acid system are schematically illustrated in Fig. 6. In the presence
of kaolinite, amino acids will be picked up from an aqueous solvent
and brought into solid solution, Amino groups become hydrogen bonded
to structural oxygen, or in the case of basic amino acids, occur
as positive ions. They are tightly fixed to the silicate surface,
and thus rendered inactive. Carboxyl groups associated with charged
Al-oxy-hydroxy groups by means of ionic bridges become directly
attached to the aluminum. In water, amino acids cannot polymerize
because of dipole-dipole interactions. In solid solution, however,
amino acids will polymerize, because the solvent medium does not
interfere, and because 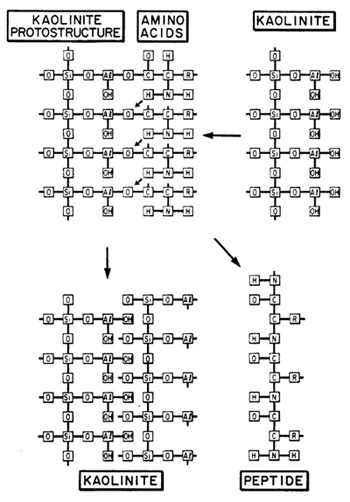
Considering the complexity of life it may come as a surprise that
only about 300 monomeric building blocks, lots of water, and some
salt are needed to generate a]] the vital stuff in the genetic and
meta bolic apparatus. However, this stuff has to become neatly packaged
into a cellular envelope in a manner able to fire the engine of
life. So it seems that the most critical question has to do with
the way membranes arise and of how that system is energized and
autocatalytically maintained (evolution is hereby of no relevance
because thermodynamically it constitutes just a disorder phenomenon).
The answer is simple, life has adopted biophosphates for various
structural and functional assignments. In the biological sciences
a quiet revolution is presently going on, namely the recognition
that the architectural principles observed in biophosphates are
identical to those established in the inorganic phosphates. The
early adoption of the name phosphorus, the carrier of light, has
not lost its true meaning over the centuries. Phosphorus, in the
form of phosphate bonds, is the carrier of energy in the living
system. It is the "energy currency" as George Wald so nicely put
it, which becomes printed, exchanged, and converted at various rates
in the perpetuating cycle of life. It is phosphorus which controls
the structure and shape of cellular material and which thus selects
the energy transfer sites. Thus, in the element phosphorus lies
the answer to the question of what distinguishes life from an ordinary
mineral. Life is based on PO 4 units, and rock-forming minerals
on SiO4 units:  It is essentially the pi-electron the high energy bond - in PO4 which maintains the animated world. Since the 7t bond can lie "parallel" to any of the four sigma bonds, giving rise to a variety of differently shaped tetrahedra, a flexible and dynamic tetrahedra network can be created. It is principally the type of metal ion adopted by PO4 that shapes the tetrahedron. In contrast, the SiO4 unit has "just" four sigma bonds which only per 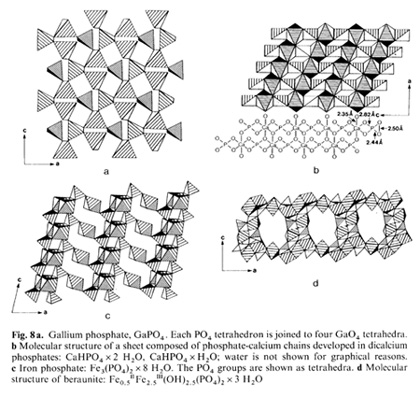 mit the establishmcnt of rigid networks, How can we possibly imagine such networks to look alike? To overcome difficulties in visualizing order phenomena, for instance, in biological membranes, examples of known layered phosphate structures in ordinary minerals are presented for illustration (Fig, 8). Phosphate tetrahedra and metal ion oxygen polyhedra can combine to a variety of geometries including undulating surfaces or concave/convex perforated surfaces, These loosely arranged space fabrics exhibit selective molecular sieve properties and ion exchange characteristics, Phospholipid membranes are expected to exhibit identical properties and structures as they exist in inorganic phosphate crystals, even including holes and surface granularities, It is proposed that the interchangeable nature of metal ions causes membranes to act as dynamic molecular sieves, Their pore size and shape must be quite variable as a function of type and availability of metal ions which in the last instances are enzyme controlled, Assuming adenosine triphosphate (ATP) is capable of trapping metal ions but adenosine diphosphate (ADP) is not, a periodic pulsation of the membrane lattice is the consequence, What we learned in this brief discussion on the structural and functional relationship between inorganic and biotic phosphate "membranes" may now permit us to understand better the mechanisms behind the origin of cellular structures at the dawn of time. It all has to do with the ability of phospholipids to jointly with metal ions construct stable fabrics and become separated from the aqueous medium. Experimental data on emulsions and foams show that micelles equipped with anisotropic membranes are able to grow at the expense of other micelles by consuming them through surface attachment (lowering in surface energy) in a process called emulsification. These globules, soaps, or emulsions as they are termed exhibit an optimal critical diameter in the order of 103 to 104 A. The main feature of this water-organic system is an anisotropic and charged phase boundary layer. The newly generated macromicelles created in a process termed coacervation, will envelop water droplets, whereby the original micelle content is exchanged but according to laws different from those established in aqueous systems. That is, condensation of lipid membranes towards a rigid membrane is achieved by the uptake, for instance, of cholesterol or metal ions. The expulsion of water proceeds during intercellular attachment by means of oxygencoordinated metal bridges. Due to the ionic fabric closely attached to the coacervate, dissolved organic molecules such as peptides or carbohydrates are bonded and precipitated on the membrane surface. In the course of coacervate development structures will arise which are enclosed by a double phospholipid skin (bilayer membrane). Phosphate groups become orientcd towards the aqueous phase and double layers may combine to multilayered stacks. Membrane pouches come into existence, resulting in the for mation of multichambered coacervates bearing striking resemblance to mitochondria. Judged by the conservative nature of mitochondria, it appears that, as a system, it still carries relics of its abiotic origin. The development of such a selfcontrolled reaction agrees with the thermodynamics of system behavior. A stable cyclic process can exist in the vicinity of a stationary phase and may operate repeatedly an infinite number of times without ever passing through the stationary phase itself. In conclusion, the primordial metabolism of the coacervate was in all probability maintained by means of a reversible phosphorylation cycle. In consequence, the origin of metabolism is in no way linked to the development of the genetic transcription apparatus. It must be considered an independent formation process. For this reason the abiotic origin of phosphorylation must be regarded as an equally important step towards the creation of the primordial cell. The problem, therefore, centers around the question of how to polymerize the common monophosphates into di-, tri-, or tetraphosphates, since polyphosphates are unstable in natural environments. The only reasonable choice left is to place the polymerization event within the coacervales. It is conceivable that phospholipid solid-state surfaces served in this capacity, because inorganic mineral surfaces too act as templates and furthermore may catalyze phosphorylation as has been demonstrated for apatite crystals. The establishment of an interconnected and chemical reaction pattern for the coacervate system as a whole exists when phosphorylation can be maintained. This requires a constant supply of organic molecules and metal ions that are consumed, or utilized during this development. In this manner, a certain modus vivendi is established. Sources of energy were oxidizable organic compounds in the ambience. Molecules such as amino acids or sugars must have been present in huge quantities in the environment in view of their mineral fabricated origin. So far, however, no vital power was involved. It is postulated that a primitive heterotrophic metabolism improved progressively. Its development took shape independently of the evolution of the nucleic acids and the genetic code. By superimposing the two separately developed entities, (a) the genetic apparatus, and (b) the heterotrophic metabolism, the primordial cell came into existence. 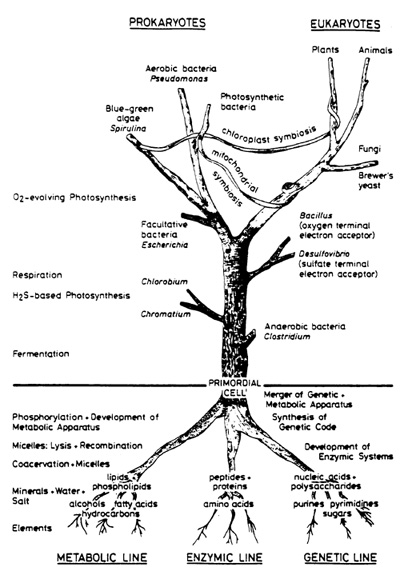
We have come a long way during this presentation. I have "crudely" abstracted from the wealth of data available on the origin of the first living cell, but still hope that my ""nutshell" approach has provided at least some idea of the work being done in a field of science involved with unraveling the mysteries of life. A more comprehensive treatise may be consulted (Degens 1989) for special references or to obtain further details on the roots and evolution of the biological cell in the course of more than 4 billion years of Earth's history.
Degens ET (1989) Perspectives on biogeochemistry. Springer, Berlin
Heidelberg New York, pp 423 |