In: Zander AR et al. (eds) Gene Technolgy, Stem Cell and Leukemia Research, Nato ASI Series H: Cell Biology, Vol 94, Springer-Verlag, Berlin Heidelberg New York London, pp 295-306 |
|
Department of Cell Biology DNAX Research Institute of Molecular and Cellular Biology 901 California Avenue Palo Alto, CA 94304 USA
Hematopoiesis is a complexed process of cell proliferation and differentiation that are regulated by a number of cytokines (Arai et al., 1990). Among such cytokines, interleukin-3 (IL-3) stimulates multipotential hematopoietic progenitors as well as lineage-committed cells including granulocytes, macrophages, megakaryocytes, erythroid cells, and mast cells, resulting in formation of the colonies of multiple lineages. Thus, IL-3 is also known as a multi-colony stimulating factor (multi-CSF). Granulocyte macrophage colony stimulating factor (GM-CSF) stimulates not only granulocytes and macrophages, but also various hematopoietic cells and exhibits activities similar to IL-3. In contrast, IL-5 has a narrow target cell specificity and mainly stimulates eosinophils and CD5-positive B cells. Interestingly, in factor-dependent cultured cells, these three cytokines are strong mitogens which induce almost identical biochemical responses such as tyrosine phosphorylatin of intracellular proteins and activation of signaling pathways (Miyajima et al., 1993). Although IL-3, GM-CSF, and IL-5 show an overlapping biological activity on a certain type of cells (e.g. eosinophils), both GM-CSF and IL-5 do not possess multi-CSF activity on the primitive progenitor cells and mast cell stimulation activity. Biological activities of IL-3, GM-CSF and IL-5 are mediated by their specific high affinity receptors which consist of alfa and ß subunits, members of the class I cytokine receptor family (Miyajima et al., 1993). The alfa subunits are glycoproteins of 60- 70 kilodalton (kD) and bind their specific ligand with low affinity. Reconstitution experiments have shown that the high affinity receptors for IL-3, GM-CSF, and IL-5 share the ß subunit (ßc) that is a glycoprotein of 120130 kD. The ßc does not bind any cytokine by itself, but forms high affinity receptors with anyone of the three alfa subunits: i. e. IL-3Ralfa , GM-CSFR alfa , and IL-5R alfa . Thus, the alfa subunits determine the cytokine specificity of the high affinity receptors. When cytokine and the alfa and ß subunits form a complex on the cell surface, various intracellular signaling cascades are activated. Although intracellular domains of both alfa and ß subunits are required for transmitting a proliferation signal (Takaki et al., 1994 ), the ß subunit plays a major role in signaling including Ras/Raf activation, myc induction, and JAK2 kinase activation (Sato et al., 1993; Quelle et al., 1994). Thus the common biological responses are elicitted by IL-3, GM-CSF, and IL-5 through the common signal transducer, ßc. It should be noted that in mice there are two homologous ß subunits, ß c and IL-3 specific ß, ßIL-3, which binds IL-3 with low affinity and form a high affinity receptor with only IL-3R alfa . The two high affinity IL-3Rs formed with either ßc or ßIL-3 show any functinal differences (Takaki et al., 1991; Hara and Miyajima, 1992; Park et al., 1992). Expression of alfa and ß subunits of IL-3/GM-CSF/IL-5 receptors is mainly restricted in hematopoietic cells. The ß subunits are expressed in myeloid progenitor cells, macrophages, mast cells, and CD5-positive B cells, but not in T cells and fibroblasts (Gorman et al., 1990). In contrast, expression of the alfa subunits is restricted to each cytokineresponsive cells (Hara and Miyajima, 1992). To better understand the function of each subunit in hematopoietic cell development, we have studied mice that show altered expression of IL-3R alfa and IL-5R alfa. In this article, we describe recent findings on the role of the IL-3R alfa subunit and the IL 3/IL-3R system in hematopoiesis. In the bone marrow, primitive hematopoietic progenitor cells are
self-renewing and they are capable of differentiating into various
lineage of mature hematopoietic cells upon stimulation with IL-3.
Such a multipotent progenitor population can be isolated by using
antibodies against several cell surface antigens (Heimfeld et al.,
1991). As we expected, both alfa and ß subunits (ßc and ßIL-3) of
IL-3R are expressed in the multipotential progenitor cells (Fig.
1 ). 
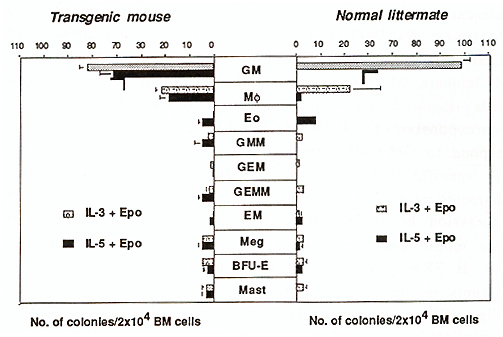
So far as we know, IL-3 is the only cytokine which strongly stimulates multipotential hematopoietic progenitor cells. Hence it is of interest to know whether hematopoiesis is affected in mice lacking the functional IL-3R. Interestingly, IL-3 nonresponsive mice have been reported (Morris et al., 1990) and recently we found that expression of IL-3R in these mice are impaired (Ichihara et al., 1994; T. H., M. Ichihara, M. Takagi, and A. M., submitted). Bone marrow cells isolated from these mouse strains (A type) are nonresponsive to IL-3 in colony assays, however, they respond to GM-CSF normally, indicating that they lack an ILR specific component or signaling molecule. Detailed characterization has revealed that they are indeed deficient in expression of IL-3R alfa (Fig. 3A). The IL-3-nonresponsive A type mouse strains possess a common mutation in intron 7 of the IL-3R alfa gene. This mutaion, a 5 base-pairs deletion, disrupts the branchpoint consensus sequence for RNA splicing (Fig. 3B ). Consequently, the alternatively spliced IL-3R alfa transcript is produced in A type mice. Since the IL-3R alfa protein produced from an alternatively spliced RNA lacks exon 8 encoding10 amino acid residues in the extracellular domain, it is discriminated from the normal product and is localized intracellularly but not translocated to the cell surface. The IL-3-nonresponsive mouse strains thus do not express a normal level of IL-3R alfa nor high affinity IL-3Rs. Remarkably, we have found 10 IL-3-nonresponsive A type mouse strains out of 27 inbred mouse strains (Table 1). The A type mouse strains do not apparently have a defect in constitutive hematopoiesis nor disadvantage In forming mouse colonies. Thus the A type IL-3R alfa gene appears to be an allele which is not eliminated from the mice population in the laboratory settings. Interestingly, however, only 1 out of 21 wild-derived mouse strains we examined carry the A type IL-3R alfa gene (T. H., M. Ichihara, M. Takagi, and A. M., submitted). This result may indicate some selective advantage of mice that possess the IL-3/IL-3R system in wild populations. Table 1. Genotype of IL-3Ralfa
gene in inbred mouse strains  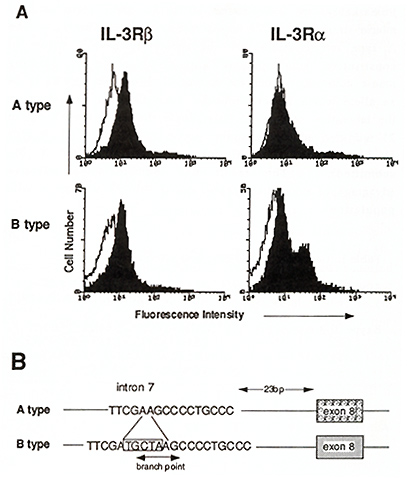
The authors would like to thank Drs. S. Hudak, D. Rennick, M. Takagi,
and M. Ichihara for their help
Arai, K., Lee, F., Miyajima, A., Miyatake, S., Arai, N., and Yokota, T. (1990). Cytokines: coordinators of immune and inflamatory responses. Annu. Rev. Biochem. 59 : 783-836 Gorman, D. M., Itoh, N., Kitamura, T., Schreurs, J., Yonehara, S., Yahara, I., Arai, K., and Miyajima, A. (1990). Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc. Natl. Acad. Sci. USA 87: 5459-5463 Hara, T., and Miyajima, A. (1992). Two distinct functional high affinity receptors for mouse IL-3. EMBO J. 10: 1875-1884 Heimfeld, S., Hudak, S., Weissman, I., and Rennick, D. (1991). The in vitro response of phenotypically defined mouse stem cells and myeloerythroid progenitors to single or multiple growth factors. Proc. Natl. Acad. Sci. USA 88: 9902-9906 Ichihara, M., Hara, T., Takagi, M., Cho, L. C., Gorman, D. M., and Miyajima, A. (1994 ). Defective interleukin-3 (IL-3) response of the A/J mouse is caused by a branch point deletion in the IL-3 receptor alfa subunit gene. EMBO J. in press Longmore, G. D., Pharr, P. N., and Lodish, H. F. (1994). A constitutively activated erythropoietin receptor stimulates proliferation and contributes to transformation of multipotent, committed nonerythroid and erythroid progenitor cells. Mol. Cell. BioI. 14: 2266-2277 Miyajima, A., Mui, A. L., Ogorochi, T., and Sakamaki, K. (1993). Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood 82: 1960-1974 Morris, C .F., Salisbury , J ., Kobayashi, M., Townsend, P. V ., and Hapel, A. J. ( 1990). Interleukin 3 alone does not support the proliferation of bone marrow cells from A/J mice: a novel system for studying the synergistic activities of IL-3. Br. J . Haematol.74:131-137 Park, L. S., Martin, U., Sorensen, R., Luhr, S., Morrissey, P. J., Cosman, D., and Larsen, A. (1992). Cloning of the low-affinity murine granulocyte-macrophage colony-stimulating factor receptor and reconstitution of a high-affinity receptor complex. Proc. Natl. Acad. Sci. USA 89: 4295-4299 Quelle, F. W., Witthuhn, B. A., Inhorn, R., Ernst, T. J., Miyajima, A., Griffin, J. D., and I hIe, J. N. (1994). JAK2 associates with the ßc chain of the receptor for GM-CSF and its activation requires the membrane proximal region. Mol. Cell. BioI. 14:4335-4341 Sato, N., Sakamaki, K., Terada, N., Arai, K., and Miyajima, A. (1993). Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 12:4181-4189 Takagi, M., Hara, T ., Ichihara, M., Takatsu, K., and Miyajima, A. (1994). Multi-colony stimulating activity of interleukin-5 (IL5) on hematopoietic progenitors from transgenic mice that express IL-5 receptor alfa subunit constitutively. J. Exp, Med. i n press Takaki, S., Kanazawa, H., Shiba, M., and Takatsu, K. (1994). A critical cytoplasmic domain of the interleukin-5 (IL-5) receptor alfa chain and its function in IL-5-mediated growth signal transduction. Mol. Cell. BioI. 14:7404- 7413 Takaki, S., Mita, S., Kitamura, T., Yonehara, S., Yamaguchi, N., Tominaga, A., Miyajima, A., and Takatsu, K. (1991). Identification of the second subunit of the murine interleukin-5 receptor: interleukin-3 receptror-like protein, AIC2B is a component of the high-affinity interleukin-5 receptor. EMBO I. 10: 2833-2838 |