|
Department of Clinical Physiology Clinical Research Centre University of Ulm 79 Ulm, Parkstrasse 11
The diffusion chamber technique was originally developed by Algire ( 1) for investigation of immunological problems and was then used by other workers for the isolated growth of various tissues under in vivo conditions. Later the technique was applied to the growth of haematological cells (2) and developed as a quantitative test system for haematological stem cells by Benestad, Böyum and Breivik (3,4,5). Since most assays for haematological stem cells are limited to laboratory animals, the importance of the diffusion chamber method is that it can be applied to test for human stem cells. With the in vitro agar colony technique, the myelopoietic committed stem cell can be tested ( 6) and with the plasma clot cultures also the erythropoietic committed stem cell ( 7) .The diffusion chamber technique provides a milieu in which the pluripotent stem cell can proliferate (5,8) and differentiate since all three cell series -myelopoiesis, erythropoiesis and megakaryocytopoiesis are found in cultures ofhuman bone marrow cells (9) and of peripheral blood cells (10) in diffusion chambers.
The principle of the method is as follows. The chamber is usually built from a plexiglas ring, whose sides are closed by two MF-Millipore filters, pore size 0.22 µ or 0.45 µ through which nutrients can diffuse. After sterilisation, the chambers are filled through a hole in the ring with the cell suspension to be studied, sealed and implanted into the peritoneal cavity of normal or pre-treated animals. The chambers can be harvested at different intervals, and are shaken in medium containing 0,5% pronase to disperse the clot cells which has formed (unless histological sections are desired). The cell suspension is then removed from the chamber by puncture with a fine Pasteur pipette and, after washing out the chamber, transferred to a small centrifuge tube where the total volume is found by weighing. The total cell number can then be determined and smears made from the cell pellet after centrifugation. We are using this system to study the growth of peripheral blood cells from normal subjects or leukaemic patient. Human peripheral blood was chosen in preference to bone marrow, since it is easily available and especially because the cell population to be implanted can be confined to about 99% mononuclear cells (lymphocytes and monocytes) by removal of granulocytes and erythrocytes on an Isopaque-Ficoll gradient (11 ). The subsequent appearance of immature precursor cells in the chambers can then be taken as evidence of their development from stem cells and there is no confusion with the possible survival of immature cells present in the original inoculum. Furthermore, a possible stimulatory (12) or inhibitory (13) effect of granulocytes on cell growth is excluded so that a cell inoculum of more constant composition is implanted which is subject only to stimulation by factors in the host animal which can be maintained constant or varied at will.
The growth of cells when normal mononuclear blood cells are implanted into mice pre-irradiated with 650 R has the following course (Figure 1). There is an initial decrease in cell number to 70% to 80 %, owing to the technique and to cell death, followed by an increase after the 6th day, reaching a maximum on the 13th day of at least twice the previous minimum. As soon as 24 hours after implantation, immature blast cells can be observed. An increase in these blast cells follows and myelopoietic precursors appear after the sixth day. Erythropoietic precursors are very few in comparison and megakaryocytes of different maturation stages appear from the ninth day onwards.
The following questions in human acute leukaemia are presently
being investigated: 1. What is the proliferative capacity of cells
from patients with acute leukaemia? Is there any relationship between
the capacity for proliferation of leukaemic cells and the type and/
or stage of the leukaemia ? Up to know, the proliferation and differentiation
of leukaemic cells has been investigated by several groups using
the agar colony technique. However, the agar colony technique is
a test for the stem cell committed to myelopoiesis. Since the leukaemic
lesion probably lies at an earlier stage, that is at the level of
the pluripotent stem cell, it is desirable to use in addition a
method such as the diffusion chamber technique which permits the
proliferation and differentiation of cells at this level of development.
In all patients, therefore, mononuclear blood cells separated on
an Isopaque-Ficoll gradient were tested for their growth potential
in both the in vivo diffusion chamber technique and the in vitro
agar technique. In the diffusion chamber experiments, the growth
pattern of cells from patients with acute leukaemia shows wide variations
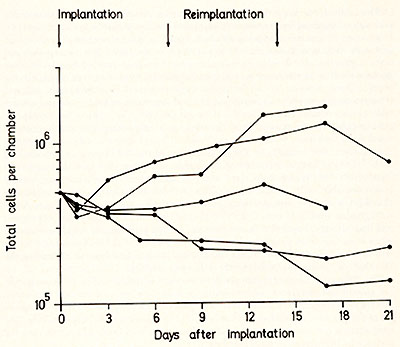
compared with the normal pattern (Fig. 1 ), as is illustrated by
the examples given in Fig. 2. In some cases, the increase in cell
number may be more than twice that from normal cells, in some cases
it is similar to normal and in other cases there may be little or
no growth at all. The relevance of these data to the type and stage
of the disease, or to the prognosis cannot be assessed at present
since too few cases have been investigated. However, the varying
growth of leukaemic cells when brought into a "normal" milieu shows
that they have different proliferative capacities. 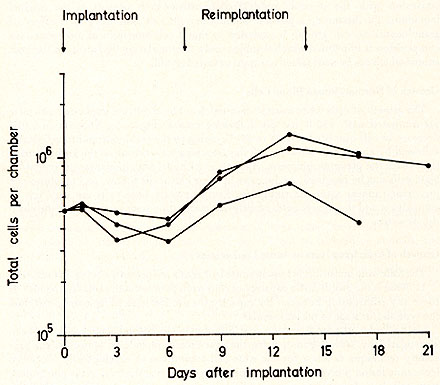
In comparing the growth of cells in the two systems, there are again wide variations. In some patients the leukaemic cells show neither growth in diffusion chambers nor colony formation in agar culture, whereas in others exceeding growth and some maturation in diffusion chambers is associyted with an abundant but diffuse growth in agar culture with only small cluster formation. An important finding is that in some cases growth in the in vivo and in vitro systems is quite different, with poor growth in diffusion chambers but good colony formation in agar culture. Such a difference in growth could arise because different stimulatory or inhibitory factors are provided in the in vivo and the in vitro milieu thereby probably supporting the growth of different types of cells. The second question we are interested in is: 2. Do leukaemic blast cells possess a capacity to differentiate similar to that of normal pluripotent stem cells, or of different unipotent committed stem cells, i. e. can they in a "normal" milieu produce recognisable precursor cells of the myelopoietic, erythropoietic and megakaryocytic series ? That cells from some leukaemic patients can mature along the myelopoietic pathway is suggested by the observation of Auer bodies in mature granulocytes (14 ). Maturation of leukaemic cells has also been found in agar cui ture ( 15, 16) and of rat leukaemic cells in diffusion chambers ( 17) .We have also found that myelopoiesis and mature granulocytes develop in diffusion chambers in growth of cells from some patients studied. In two cases mature megakaryocytes were observed. An underlying problem here, however, is the distinction between growth from leukaemic cells and from remaining normal cells, which necessitates separation of leukaemic and normal cells. Since a reliable method for this does not yet exist, the problem can perhaps be circumvented in various ways: 1) by choosing for investigation patients with very high numbers of leukaemic blast cells in the blodd, 2) by cytogenetic analysis for common chromosome abnormalities or 3) by pre-Iabelling of the cell suspension. Pre-Iabelling with 3 H-thymidine in vitro seems profitable since very few normal blood cells incorporate 3 H-thymidine in vitro and the labelled cells are almost exclusively leukaemic blast cells, so that the occurrence of labelled maturing cells in diffusion chambers would be strong evidence for their leukaemic blast cell origin. Autoradiographic studies of cells from 12 leukaemic patients investigated are in progress and have already shown that in one patient, labelled granulocytes could be observed when blast cells from peripheral blood were pre-Iabelled with 3 H-thymidine. Further results should show whether or not this method is a practicable mean of deciding the "normal" or "leukaemic" origin of the differentiated cells. The diffusion chamber technique provides further the possibility of investigating cell growth and maturation under conditions where the levels of humoral regulatory factors are changed, for instance after intraperitoneal administration of erythropoietin or factors which stimulate ( 18) or inhibit ( 19) colony growth in agar culture. The host animals can also be treated to alter the endogenous levels of such factors, for instance by hypertransfusion of bleeding ( 6 ). In conclusion, the comparison of growth of leukaemic cells in diffusion chambers and in agar culture should reveal what proliferative and differentiating capacity the leukaemic cells have. Furthermore, it is hoped that such studies could lead to a functional classification with respect to the different types and stages of human acute leukaemia.
The excellent technical assistance of Mrs. U. Ertl, Mrs. A. Milewski and Mrs. I. Dittmann is gratefully acknowledged. The investigation was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 112) "Zellsystemphysiologie" ) and by EURA TOM Contract No.088-72-1 BIAC.
1. Algire, G. H.: Ann. N. Y. Acad. Sci, 69,663-667 (1957) 2. Berman, I. and Kaplan, H. S.: Blood 5,1040-1046 (1959) 3. Benestad, H.: J. Haemat. 7, 279-288 (1970) 4. Böyum, A., and Borgström, R.: Scand. J. Haemat. 7,294-303 (1970) 5. Breivik, H., Benestad, H. B. and Böyum, A.: J. Cell. Physiol., 78, 65- 72 ( 1971) 6. Haskill, J. S., McNeill, T. A. and Moore, M. A. S.: Density distribution analysis of in vivo and in vitro colony forming cells in bone marrow. 7. Stephenson, J. R., Axelrad, A. A., McLeod, D. L. and Shreeve, M. M.: Proc. nat. Acad. Sci. 68, 1542-1546 (1971) 8. Tyler, w. S., Niskanen, E., Stohlman, F., Keane, J. and Howard, D.: Blood 40, 634-645 (1972) 9. Böyum, A., Boecker, W., Carsten, A. L. and Cronkite, E. P.: Blood40, 163-173 (1972) 10. Boecker, w. R., Böyum, A., Carsten, A. L. and Cronkite, E. P.: Blood 38,819 (1971) 11. Böyum, A.: Scand. J. Clin Lab. Invest. 21 Suppl. 97 (1969) 12. Robinson, w. A. and Mangalik, A.: Lancet, ii, 742-744 (1972) 13. Paran, M., Ichikawa, Y. and Sachs, L.: Proc. nat. Acad. Sci.: 62,81-77 (1969) 14. Leder, L. D.: Acta haemat., 42,58-63 (1969) 15. Paran, M., Sachs, L., Barak, Y. and Resnitzky, P .: Proc. nat. Acad. Sci. 67, 1542-1549 (1970) 16. Greenberg, P. L., Nichols, w. C. and Schrier, S. L.: New Engl. J. Med.284, 1225-1232 (1971) 17. Parrish, w. and Kleinfeld, R. G.: Cancer Res., 23,1164-1168 (1963) 18. Metcalf. D.: Austr. J. exp. Biol. med. Sci. 50, 547 -557 (1972) 19. Chan, S. H. and Metcalf, D.: Nature 227,845-846 (1970) |