In: Zander AR et al. (eds) Gene Technolgy, Stem Cell and Leukemia Research, Nato ASI Series H: Cell Biology, Vol 94, Springer-Verlag, Berlin Heidelberg New York London, pp 353-360 |
|
Dept. of Immunohaematology and Blood Bank, University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands.
Several reports demonstrated the presence of anti-host mHag specific CTL in patients suffering from GvHD after HLA genotypically identical BMT (Goulmy, 1983; Tsoi, 1980, 1983; Irle, 1985; Van Els, 1990; Irscheck, 1992; Niederwieser, 1993) .In our laboratory , much effort was put into the further characterization of a (small) number of anti-host mHag specific CTLs. Hereto, CTL clones specific for host mHag were isolated from the peripheral blood (PBL) of patients suffering from severe GvHD. Subsequent immunogenetic analyses revealed that these CTL clones identified five non-sexlinked mHag, designated HA-1, -2, -3, -4, -5, which are recognized in a classical MHC restricted fashion (Van Els, 1992) .mHag HA-3 is recognized in the presence of HLAA 1 and mHag HA-1 , -2, -4 and 5 require the presence of HLA-A2. In order to document the effect of mH antigens in genotypically identical BMT on the occurrence of acute (grade >= 2) GvHD, we prospectively collected PBL from HLA-A 1 and HLA-A2 positive patient/donor sibling pairs. This multi center study comprised 148 HLA genotypically identical BM donor/recipient combinations, adults as well as children, grafted between 1982 and 1990. The results of the mHag typing using the CTL clones specific for five well defined mHag HA-1 to HA-5 demonstrated (table 1) a significant correlation between mHag HA-1, -2, -4 and -5 mismatch and GvHD (Goulmy, 1994). Table 1 Correlation of mHag HA-1. -2.
-4 and -5. with the occurrence of GvHD 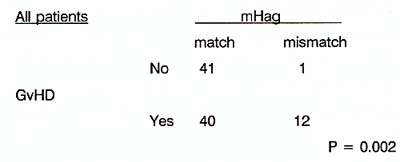
The hypothesis that posttransplantation of bone marrow anti-host CTL activity may have a beneficial effect is based on the assumption of the postulated anti-Ieukaemic potential as a 'desired' side-effect of the post BMT complication GvH. (Bortin, 1973; Weiden, 1981 a, 1981 b). In search for anti-host CTL and Th cell activities post BMT, we observed earlier both absence and presence of anti-host CTL in patients without any clinical signs of GvHD (see table 2). Table 2 Anti-host T cell activities after
HLA identical BMT. 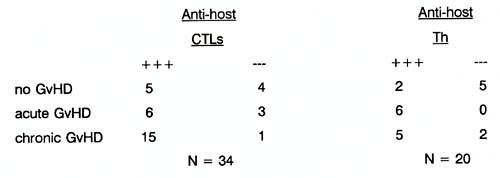
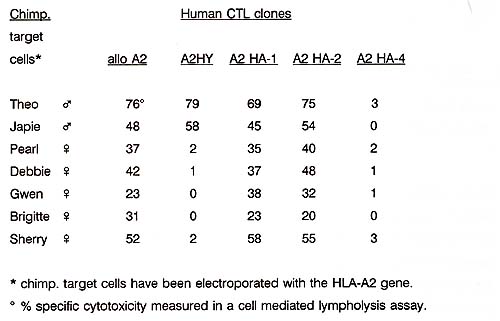
To substantiate the importance of the human mH antigenic systems, we investigated whether the mHag are conserved in evolution between man and chimpansee. Hereto, cells from chimpansees were transfected with the human HLA-A2.1 gene. Subsequent analyses with our human allo HLA-A2.1 and four mHag HLA-A2.1 restricted CTL clones revealed the presentation of chimpansees' allo and mHag peptides in the context of the transfected human HLA-A2.1 molecule by chimpansees' target cells (table 3) .These results implicate that the chimpansee cell derived allo and mHag peptides investiged in this study are very similar to the human allo HLA-A2 and HLAA2 restricted mHag peptides. Table 3 Human mHag are evolutionary conserved
I am indebted to Els Blokland, Ronald Bontrop, Cecile van Els, Fred Falkenburg, Ellen van Lochem and Jos Pool for their great contributions. Ingrid Curiel for typing the manuscript. This work was supported in part by grants from the Dutch Organisation for Scientific Research (NWO), the J.A. Cohen Institute for Radiopathology and Radiation Protection IRS) and the Dutch Cancer Society.
Beatty PG, Herve P (1989) Immunogenetic factors relevant to acute GvHD. In: S.J. Burakoff, D.H.J. Deeg, S. Ferrara, K. Atkinson (eds): Graft-versus-Host-Disease, Immunology, Pathophysiology and Treatment. New York, Dekker, 415-23. Bortin MM (1970) A compendium of reported human bone marrow transplants. Transplantation, 9: 571-587. Bortin MM, Rimm AA, Salzstein EC, Rodey GE (1973) Graft versus leukemia. III. Apparent independent anti-host and anti-leukemic activity of transplanted immunocompetent cells. Transplantation 16: 182-188. Bortin MM, Horowitz MM, Ursic M, Rimm AA and Sobocinskym KA (1991 ). Progress in bone marrow transplantation for leukemia: a preliminary report from the advisory committee of the international Bone Marrow Transplant Registry. Transplant Proc. 23: 61-62. Falkenburg F, Goselink H, van der Harst D et al (1991 ). Growth inhibition of clonogenic leukemic precursor cells by minor histocompatibility antigen-specific cytotoxic T lymphocytes. J. Exp. Med. 174: 27-33. Goulmy E, Schipper R, Pool Jet al. Minor histocompatibility antigen mismatches influence the development of GvHD after HLA genotypically identical bone marrow transplantation. Manuscript subm. for publication 1994. Goulmy E, Termijtelen A, Bradley BA, Van Rood JJ (1977). Y-antigen killing by T cells of women is restricted by HLA. Nature 266: 544-545. Goulmy E, Gratama JW, Blokland E, Zwaan FE, van Rood JJ (1983) A Minor transplantation antigen detected by MHC restricted cytotoxic T lymphocytes during graft-versus-host-disease. Nature 302: 159-161. Irle C, Beatty PG, Mickelson E, Thomas ED, Hansen JA (1985) Alloreactive T cell responses between HLA identical siblings. Transplantation 40: 329-333. Irle C, Chapuis B, Jeannet Met al (1987) Transplant Proc. suppl. 1, 19: 2674. Irscheck E, Hladik T, Niederwieser D et al (1992) Studies on the mechanism of tolerance or Graft-versus-Host Disease in allogeneic bone marrow recipients at the level of cytotoxic T cell precursor frequencies. Blood 79: 1622-1628. Martin PJ (1991 ). Increased disparity for minor Histocompatibility antigens as a potential cause of increased GvHD risk in marrow transplantations from unrelated donors compared with related donors. Bone Marrow Transplantation. 8: 217-223. Niederwieser D, Grassegger A, Auböck J, Herold M, Nachbaur D, Rosenmayr A, Gächter A, Nussbaumer W, Gaggl S, Ritter M and Huber C (1993) Correlation of minor histocompatibility antigen specific cytotoxic T lymphocytes with Graftversus-Host Disease status and analyses of tissue distribution of their target antigens. Blood, 81: 2200-2208. Reinsmoen NL, Kersey JH, Bach FH (1984). Detection of HLA restricted anti minor histocompatibility antigen(s) reactive cells from skin GvHD lesions. Human Immunol. 1, 11: 249-257. Schwarer AP, Jiang JZ, Barrett JM et al (1993). Helper T -lymphocyte precursor (HTLp) frequency predicts the occurrence and severity of acute GvHD and survival after allogeneic BMT in both recepients of genotypically HLA-identical sibling (SIB) and phenotypically HLA-matched unrelated donor (MUD) marrow. Lancet 341: 203-205. Theobald M, Nierle T, Bunjes D et al (1992). Host-specific interleukin-2-secreting donor T cell precursors as predictors of acute Graft-versus-Host Disease in bone marrow transplantation between HLA-identical siblings. N. Engl. J. Med. 327: 1613-1617. Tsoi M-S, Storb R, Dobbs S, Medill I, Thomas ED (1980). Cell mediated immunity to non-HLA antigens of the host by donor lymphocytes in patients with chronic graft-vs-host disease. J. Immunol. 125: 2258-2262. Tsoi M-S, Storb R, Santos E, Thomas ED (1983) Anti-host cytotoxic cells in patients with acute graft-versus-host disease after HLA identical marrow grafting. Transplant Proc. 15: 1484-1486. Van der Harst D, Goulmy E, Falkenburg JHF et al (1994). Recognition of minor histocompatibility antigens on lymphocytic and myeloid leukemic cells by cytotoxic T -cell clones. Blood 83: 1060-1 066. Van Els C, Bakker A, Zwinderman AH, Zwaan FE, van Rood JJ, Goulmy E (1990a) Effector mechanisms in GvHD in response to minor Histocompatibility antigens. I. Absence of correlation with CTLs. Transplantation 50: 62-66. Van Els CACM, Bakker A, Zwinderman AH, Zwaan FE, Van Rood JJ, Goulmy E (1990b) Effector mechanisms in GvHD in response to minor histocompatibility antigens. II: Evidence for a possible involvement of proliferative T cells. Transplantation 50: 67-71. Van Els C, Zantvoort E, Jacobs Net al (1990c). Graft-versus-host disease associated T helper cell responses specific for minor histocompatibility antigens are mainly restricted by HLA-DR molecules. Bone Marrow Transplantation 5: 365-372. Van Els C, D'Amaro J, Pool J, Bakker A, van den Elsen PJ et al (1992) Immunogenetics of human minor Histocompatibility antigens: their polymorphism and immunodominance. Immunogenetics 35: 161-165. Van Lochem E, De Gast Band Goulmy E (1992) .In vitro separation of host specific graft-versus-host and graft-versus-Ieukemia cytotoxic T cell activities. Bone Marrow Transplantation 10: 181-183. Weiden PL, Flournoy N, Sanders JE, Sullivan KM, Thomas ED (1981 a) Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant. Proc. 18: 248-251. Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED (1981 b) The Seattle marrow transplant team. Antileukemic effect of chronic graft-versus-host disease. N. Engl. J. Med. 304: 1529-1533. Zier KS, Elkins WL, Pierson GR, Leo MM (1983) The use of cytotoxic T cell lines to detect the segregation of human minor alloantigen within families. Hum. Immunol. 7: 117-129. |