|
Viral Leukemia and Lymphoma Branch National Cancer Institute National Institutes of Health Bethesda, Maryland 20014 Introduction Extensive evidence has demonstrated that type C RNA viruses are active agents in the causation of naturally occuring cancers. Type C RNA viruses are a distinct class of vertebrate viruses which share a common morphology, protein composition, and viral life cycle. They are spherical particles containing a large single-stranded RNA as their viral genome complexed with a RNA-directed DNA polymerase (reverse transcriptase) in a central, symmetric, electron dense core surrounded by a unit membrane. During viral replication the nucleoid condenses beneath the surface of the cytoplasmic cell membrane with subsequent "budding" of the virus from the cell surface. Type C RNA viruses have been isolated from many vertebrate species. They have been shown to cause a variety of naturally occurring vertebrate neoplastic diseases, including leukemias and sarcomas of chickens, lymphomas and related hematopoietic neoplasms and sarcomas of mice, lymphosarcomas and fibrosarcomas of domestic cats, and leukemias and sarcomas of some primates. Type C viruses have also been isolated from other mammalian species such as rats, guinea pigs, hamsters, cattle, domestic pigs, woolly monkeys, gibbon apes, and baboons (Table 1). Recently there have been reports of isolates from human tissues (see below). As yet, in some of these species, the relationship between these viruses and neoplastic diseases of their host species has not been clarified. There have also been reports of electron microscopic observations of typical type C viral particles in tissues from some other mammalian species, including dogs, horses, rhesus monkeys, and in certain human tissues, but such viruses have not yet been isolated in vitro and biochemically characterized. Type C RNA viruses exhibit varying biological activity. Some have no known pathological effect and others are extremely efficient in producing neoplasias. Also, transformation may occur either with complete or incomplete virus expression. Type C viruses have also been detected in normal tissues; embryonic and placental tissues show more type C viral expression than other differentiated tissues. The viruses produced by both normal and tumorigenic tissues are very similar to one another in their morphology, biochemical and immunological properties (1, 2). Table I: Mammalian type C RNA virus
isolates 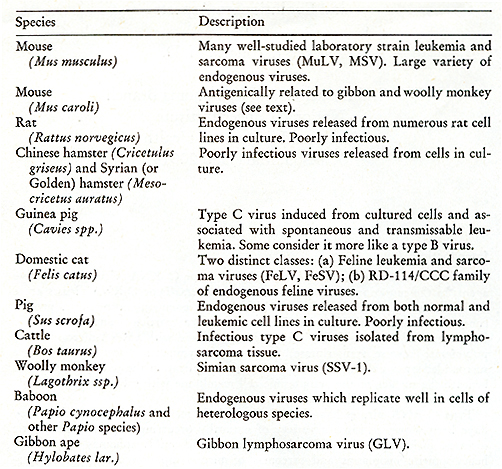
The spontaneous appearance of complete, infectious type C RNA viruses in animals of certain mammalian species and in cultured cells derived from these animals led to the hypothesis that the information for the production of such viruses might be transmitted genetically from parent to progeny along with other cellular genes (virogene-oncogene hypothesis) (3, 4). Activation of this normally repressed, genetically transmitted, type C endogenous virogene information, rather than infection from outside the animal was proposed as the most common mechanism by which type C RNA tumor viruses produce naturally occurring cancers. Table II: Species where a COMPLETE virogene
is known to be present in normal cells 
1. DNA of all somatic and germ cells of all the animals in a species contain viral gene sequences. 2. Multiple related but not identical copies present in the cellular DNA, more than DNA from a heterologous cell that is actively producing virus. 3. Virus expression (RNA, gs antigen, polymerase, complete particles) under cellular control. Expressed in certain tissues at certain times during development. 4. Clonal lines either spontaneously or after induction are capable of releasing complete virion. 5. Cells generally resistant to exogenous infection by the homologous endogenous virus.
Virogene 2. Genes maintained in population by normal cellular replication. Reverse transcriptase not required. 3. Transformation results from activation of normally latent cellular genes associated with and/or part of the viral gene sequences. Protovirus 2. Reverse transcriptase plays essen tial role in generating new viruses. 3. Transformation results from the generation of new gene sequences that do not preexist in normal cellular DNA.
as a part of the genome of type C viruses ( 4) ) are normall y repressed, bu t can be activated by a variety of intrinsic (genetic, hormonal) as well as extrinsic (radiation, chemical carcinogens, other infecting viruses) factors (Table 5). Regulatory genes and environmental factors determine the extent of virogene transcriptlon.
Virogenes 2. Type C viruses derived from closely related species should have
closely related specific antigens, e. g., gs antigens, polymerase
and their nucleic acid sequences should be more related to one another
than are those viruses released by distantly related species (virogene
evolution). 4. Spontaneous, chemically induced and viral induced transformed cells and tumor cells should have RNA as well as DNA sequences homologous to the transform ing specific sequences found in tumor viruses ( oncogene expression) .
It has only been within the last year or two that endogenous type C viruses have been successfully propagated from primates, man's closest relatives. Several isolates from different tissues and from different species of baboons have been obtained in this laboratory. They are morphologically and biochemically typical of mammalian type C viruses, are closely related by host range, viral neutralization and interference and by immunologic and nucleic acid hybridization criteria, but are distinctly different from all other previously studied type C viruses (10, 18). ³H-DNA transcripts prepared from three of the baboon type C virus isolates hybridize completely to DNA extracted from various tissues of several different healthy baboons (18). These type C virus isolates satisfy all the criteria for endogenous, genetically transmitted viruses of primates. The finding of DNA sequences in normal tissues is one of the strongest pieces of evidence that the viral information is , maintained in the population as cellular genes. J If the baboon type C viruses were truly endogenous primate viruses (10) and had )1 evolved as the species evolved, then it appeared reasonable to suspect that other Old World monkeys that are close relatives to the baboon would have related vic rogene sequences in their DNA. Primate species more distantly related taxonomi, cally to baboons would be expected to have more extensive mismatching of their virogene DNA sequences as measured by the thermal stability of nucleic acid hybrids formed or by the final extent of hybridization (19, 17). The study of the evolutionary relationships of type C viral gene sequences is especially favorable in primates since much is known about the evolutionary rela tionships between primates: the fossil record has been intensively studied as Homo sapiens have been particularly interested in their own origins. The Old World monkeys (which include the baboon species) have been separated from the great apes and man for 30 to 40 million years. The New World monkey branch diverged from the common stem leading to both the apes and the Old World monkeys, approximately 50 million years ago while the prosimians evolved from primitive mammalian stock roughly 60 to 80 million years ago. Hybridization studies employing a DNA copy of the baboon virus RNA were used to detect type C viral nucleic acid sequences in primate cellular DNA. Multiple copies of viral gene sequences related to the RNA genomes of the baboon type C viruses are found in all other Old World monkey species, higher apes, and are also found in man. However, no homology can be detected in various New World monkey DNAs (17). The degree of relatedness of the virogene sequences closely correlates with the txonomic relatedness of the monkey species based upon anatomic criteria and the fossil record. The results establish that, within the primates, type C viral genes have evolved as the species have evolved, with virogenes from more closely related genera and families showing more sequence homology than those from distantly related taxons. That such species as the baboon and rhesus monkey, which have diverged genetically and have been geographically separated for several million years, still retain related virogene sequences, and the low, but consistently observed, hybridization to ape (chimpanzee) DNA with the baboon viral probe, demonstrates that this virogene information has been conserved in the primate stock during the course of evolution as stable cellular elements for at least 30 to 40 million years (17). The ubiquitous presence of endogenous type C virogenes among anthropoid primates and their evolutionary preservation suggest that such genes provide functions with a selective advantage to the species possessing them. Virogene information is not only present in other Old World primates, but is also normally expressed. Probes from the baboon virus isolates have detected viralspecific RNA in rhesus monkey, stumptail and green monkey liver tissue; and p30 antigen has been found in normal stump tail spleen tissue and in a rhesus ovarian carcinoma (20). Two human tumors, an ovarian carcinoma and a lymphocytic lymphoma, have also been found to contain primate type C viral p30 antigen (21). These genes, therefore, are not inactive, but are normally expressed; the leve], however, varies from animal to animal and from tissue to tissue in a given animal.
Type C viruses have also, under natural conditions, been transferred between species that are only remotely related phylogenetically. In some instances, type C virogenes have escaped host control as virus particles infectious to other species. These viruses can be transmitted from one species to another with integration of their information into the DNA and subsequent perpetuation through the germ line of the recipient species. Because of the stability of the viral gene sequences when they are incorporated into cellular DNA, events that have occurred millions of years ago still can be recognized by examining the genetic information of the virus and that of the host cell. One can assess the relatedness of a given virus to the host it is associated with by comparing (using molecular hybridization) the match between the viral RNA genome and the DNA of cells from an animal of the species with which the virus is associated. Endogenous viruses from one species horizontally transmitted to another species are related to, but distinct from, one another by many different criteria: nucleic acid sequence homology, antibody inhibition of polymerase activity, antigenicity of the p30 protein, viral interference and viral neutralization. Three known examples of trans-species infections by endogenous type C genes are discussed below. One example involves the transfer of an endogenous primate type C virus into the germ line of the ancestor of the domestic cat (22, 23 ). Results have shown that domestic cat DNA contains sequences partially related to endogenous baboon type C viral sequences, even though unique sequence baboon and cat cellular DNA show no homology. Since other mammals do not contain those related sequences, the finding of baboon type C viral sequences in the distantly related domestic cat (Felis catus) cannot be explained strictly on evolutionary grounds (17). Domestic cat DNA contains type C virogenes which can lead to the production of endogenous RD-114/CCC viruses (24, 25). In comparing the endogenous primate viruses to this feline group of viruses we found that they are related to each other, but can be distinguished by biologic and immunologic criteria and by partial nucleic acid sequence homology. Endogenous viruses from one group of mammals (primates) are concluded to have infected and become apart of the germ line of an evolutionary distant group of animals, progenitors of the domestic cat (22, 23 ) and thus have had a common ancestor even though they now behave as endogenous viruses of two taxonomically distant mammalian species. Genes related to the nucleic acid of an endogenous domestic cat type C virus (RD-114/CCC) are found in the cellular DNA of anthropoid primates while at the same time many members of the cat family Felidae lack these sequences (Table 6 ).
1. The cat (RD-114/CCC) and baboon virus groups are related but
distinct from one another by:
Table VII: Examples of transmission of
type C virus genes between species 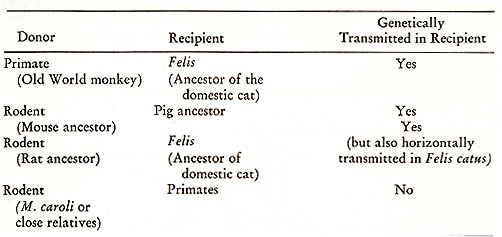
Infectious primate type C viruses have recently been recovered from several colonies of gibbon apes with various hematopoietic neoplasms, especially myelogenous and lymphoid leukemias (32), and from one woolly monkey with a spontaneous fibrosarcoma (a New World primate) (33,34). GAL V (gibbon ape leukemia virus) and SSV-SSA V (simian sarcoma virus-simian sarcoma associated virus) spread from animal to animal under natural conditions and induce tumors when inoculated into other primates (34-36). These viruses are related to one another by several immunologic criteria and contain related RNA genomes (37). Gene sequences homologous to those of the RNAs of GAL V and SSA V have not been detected in the cellular DNA of normal primates studied thus far (38, 19). Thus, unlike the baboon type C virus, these two viruses are not endogenous viruses of primates. The type C viruses of the GAL V-SSA V group are poorly controlled by the primate host and appear readily capable of producing neoplastic disease. Infection by such viruses can cause local epidemics of lymphoproliferative tumors in infected gibbon colonies (39). The ability to isolate viruses from gibbons, however, is not restricted to animals with tumors. Recently, three isolates have been obtained from the brains of normal gibbons (animals without tumors) from a single colony in the United States (37). Based on immunologic assays and interference tests, the group of infectious type C viruses of primates contains many members, all partially related to one another. At present, the infectious primate type C viruses can be classified into four distinct subgroups (see Table 8) based on hybridization studies Table VIII: Infectious primate type C
viruses; isolation and partial characterization 
Table IX: Inhibition of viral reverse
transcriptase Activity by antisera to viral polymerases 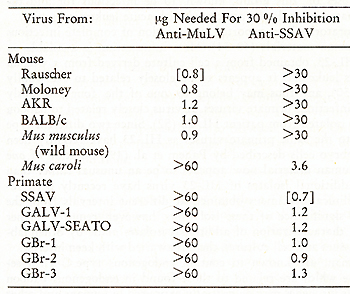
The studies of type C virogenes in primate populations as described above are unusually significant: first, they are the first isolates of type C viruses from primates; second, some of these viruses have been proven to be oncogenic; third, they provide the closest model of animal neoplasia for man; and fourth, it is possible that one, the other, or both of these two primate virus groups (GAL V and SSA V) may be involved in human neoplasia. Since the horizontally transmitted primate viruses described above are infectious for and can cause tumors in primates, the possibility exists that this group of viruses may be involved in the etiology of human cancer. This is supported by data obtained using different experimental procedures in a number of laboratories. An enzyme with biochemical properties related to those of type C viruses and with antigenic properties similar to polymerases of the woolly monkey type C virus (SSA V) and the gibbon ape leukemia virus (GAL V) has been detected in human acute leukemia cells ( 43, 44 ). The DNA products of endogenous reactions from the "virus-like" particulate fraction of acute leukemia cells hybridize preferentially to viral RNA from SSA V and GAL V (45,46). Using radioimmunoassays, antigens related to the major structural proteins (p30) of type C viruses have been detected in peripheral white blood cells from five patients with acute leukemia (47). These results suggest that viruses of this group, known to be infectious for and tumorigenic in other primates, may also be associated with acute leukemia in man. Recently, several laboratories have reported the isolation of complete infectious type C viruses from human materials ( 48-51 ). Most information is available on the isolate designated HL-23, obtained from a cell culture derived from a woman with acute myelogenous leukemia. It appears to be closely related to the woolly monkey virus, SSA V (50), and thus may belong to one of the four previously described subgroups of infectious primate viruses. A virus closely related to baboon type C viruses was also isolated from patient HL-23 (52). Since two different type C viruses also related to the same primate viruses as HL-23 have recently been found in the human embryo cells described by Panem et al. ( 49), isolation of one infectious virus from human material now appears to be an unusual rather than common occurrence. Additional isolates of HL-23 virus have recently been reported from separate clinical specimens obtained at different intervals from the same patient ( 53 ). The significance of these isolations, however, requires further evaluation. The careful characterization of additional isolates made by other laboratories from human tissues and cell cultures, then, is awaited with keen interest. Primates, including man, are known to contain endogenous type C viral sequences in their genome which are related to those found in endogenous baboon viruses (16). Endogenous virogenes may be partially expressed in humans and other primates as evidenced by the detection of RNA sequences (20), and antigens related to the p30 proteins (20, 21) of endogenous baboon viruses. The expression of endogenous viral-related antigens is found in carcinomas and lymphomas (21) as well as in leukemias (47); viral p30 antigen expression has also been reported in certain normal human tissues ( 54 ) . If infectious type C RNA viruses are important agents in cancer causation in man, it is critical to know how the viral information is transmitted, normally controlled, and maintained in the population. Are they contained in an animal reservoir or do they spread solely from primate to primate? Finding this reservoir(s), if it exists, provides a chance of disrupting the process. If human leukemia involves the spread of an infectious agent from individual to individual as is clearly shown to be the case for cat leukemia (55) and bovine leukemia (56), then identification of the agent and its mode of spread would provide one set of approaches to prevention of the disease. If, on the other hand, activation of genetically transmitted virus by extrinsic ( chemical and physical agents) as well as by various intrinsic factors leads to tumor development and there is no contagious virus involved, the approaches to the prevention of the disease would be quite different. The endogenous primate type C virogenes, present in human cells, would appear to be the more logical candidate virus for involvement in the generality of human cancer.
The presence of genetically transmitted viral genes in so many vertebrate species and the evidence that they have been conserved through evolution in several distinct vertebrate lineages suggests that they may provide normal function(s)
1. Activation of oncogenic information, while inappropriate in adult tissue, plays a normal role during differentiation and development. 2. The integrated virus serves to protect the species against related, more virulent infectious type C viruses. 3. Virus activation, being linked to transformation, protects the animal by altering the cell membrane. The released virus could alert the immune system making the transformed cells more susceptible to immunologic control. 4. They may have had an evolutionary role as conveyors of genetic information not only within a species but also between species. Only this group of viruses has been shown to transmit genes between germ cells of different species under natural conditions.
advantageous to the species carrying them (Table 10). The first suggested role, derived from studies on the expression of viral antigens during the course of development, was that such viral expression during the early stages of differentiation was a normal part of the developmental process (3). If this were the case, the expression of cancer genes later in life would be an inappropriate manifestation of a normal developmental function. If viral genes provide a function critical for normal development, they clearly would be conserved during evolution. The acquisition of viral genes by cats from both primates and rodents, and by pigs from rodents, along with the fact that they have been maintained for millions of years suggests the possibility that the newly acquired viral genes, once integrated, might have been beneficial to the recipient species if they were able to provide resistance to related, but more virulent viruses. Animals that successfully integrated the genomes would have been at a selective advantage relative to those that did not, if the integrated genome protected against infection, and if infection led to cancer or other type C viral-mediated diseases. Genes that provide protection against disease, especially against epidemic diseases, would be at a strong selective advantage in natural populations. This may well explain the success of the transmission between species as described above. For example, in our laboratory we have shown that those species of the genus Felis, including the domestic cat, that have acquired primate type C viral genes are resistant to infection by the endogenous baboon viruses, while those Felis species that have not acquired the viral information are still susceptible to baboon viral information. A third possible role for endogenous viruses arises if viral activation was closely linked to the transformed state in the cell. Expression of the endogenous virus under natural circumstances, may be protective on an immunological basis against cancer, rather than the virus acting as the etiological agent. The activated virus could alter the cell membrane and thus alert the host immune system, conveying information as to the number and location of transformed cells in the body. This possibility is supported by the observation that transformed cells in culture, whether transformed spontaneously, by chemical carcinogens, or by other viruses, release their endogenous type C viruses more readily than do their normal, untransformed counterparts (57-59). Transformed cells that are releasing high titers of type C virus have been reported to be much less able to produce tumors when inoculated into immunocompetent animals of the same species (60). Partial viral expression where viral antigens are introduced into the cell surface may be sufficient to alter its antigenicity and facilitate rejection of these cells. One final possibility that should be considered is that type C viruses have played an important evolutionary role as transmitters of genetic information, not only between cells of an animal, and animals of a species, but also between species. That viruses can transmit themselves between the germ cell DNAs of very different species has been established as a result of experiments in the past year. That they can recombine with cellular gene sequences and transmit these genes to new cells of a different species also has been clearly demonstrated (61, 62). That this transmission of cellular gene information between species has been a major force in evolution, however, remains a speculation. This suggestion that viruses may have had a major role in evolution is not anew one (63). Viruses are unique in that they can serve to carry information between genetically isolated species. Classical Darwinian evolution deals with changes which occur within the genetic information of a species; which can be changed and rearranged by mutation and selection, duplication and rearrangements, but not added to from the outside. Viruses, however, offer the possibility of additions of new gene sequences to a species. The type C viruses as a group, are uniquely suited for this role since they must incorporate into the cellular DNA in order to replicate (14) but they do not kill the cells that they infect. Each time they move from cell to cell they may carry with them host cell genes providing a means of communication between cells of different species and different phyla. They serve to keep a species in contact or in communication with its neighbors-ecologic neighbors as well as genetic neighbors. Of course they can transmit information that may disrupt normal cellular control, and by so doing, lead to the development of cancer in the individual. Instances of genetic significance, however, occur when new genes are incorporated into the germ line. From this perspective, the fact that these viruses cause cancer would then be viewed as a pathological manifestation of normal processes. While the viral genes may well be etiologic agents in cancer causation, either as exogenous or endogenous viruses, and this may be of profound significance to the affected individuals, these relatively rare and sporadic cases may not be of great evolutionary significance.
References 1. Kalter, S. S., Helmke, R. J ., Panigel, M., Heberling, R. L., Felsburg, Po J. and Axelrod, L. R. : Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science 179: 1332-1333,1973. 2. Schidlovsky, G. and Ahmed, M.: C-type virus particles in placentas and fetal tissues of rhesus monkeys. I. Natl. Cancer Inst. 51: 225-233,1973. 3. Huebner, R. Jo and Todaro, G. J.: Oncogenes of RNA tumor viruses as determinants of cancer. Proc. Natl. Acad. Sci. USA 64: 1087-1094, 1969. 4. Todaro, G. J. and Huebner, Ro J.: The viral oncogene hypothesis: New evi dence. Proc. Natl. Acad. Sci. USA 69: 1009-1015, 1972. 5. Lieber, M. M. and Todaro, G. J.: Mammalian type C RNA viruses. In: Cancer: A Comprehensive Treatise, Vol. II. Becker, F. F. (Ed.), Plenum Press, New York, 1975, pp. 91-130. 6. Lowy, D. R., Rowe, W. P., Teich, N. and Hartley, J. W.: Murine leukemia virus: High-frequency activation in vitro by 5-iododeoxyuridine and 5bromodeoxyuridine. Science 174: 155-156,1971. 7. Weiss, R. A., Friis, R. R., Katz, E. and Vogt, P. K.: Induction of avian tumor viruses in normal cells by physical and chemical carcinogenesis. Virology 46: 920-938,1971. 8. Livingston, D. M. and Todaro, G. J.: Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C viruses. Virology 53: 142-151,1973. 9. Benveniste, R. E., Lieber, M. M. and Todaro, G. J.: A distinct class of inducible murine type C viruses which replicate in the rabbit SIRC cell line. Proc. Natl. Acad. Sci. USA 71: 602-606, 1974. 10. Benveniste, R. E., Lieber, M. M., Livingston, D. M., Sherr, C. J., Todaro, G. J. and Kalter, S. S. : Infectious type C virus isolated from a baboon placenta. N ature 248: 17-20, 1974. 11. Temin, H. M.: Mechanism of cell transformation by RNA tumor viruses. Annual Review of Microbiology 25: 609-648,1971. 12. Baltimore, D. : RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 226: 1209-1211, 1970. 13. Temin, H. M. and Mizutani, S. : RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 226: 1211-1213, 1970. 14. Temin, H. M.: The RNA tumor viruses -background and foreground. Proc. Natl. Acad. Sci. USA 69: 1016-1020,1972. 15. Benveniste, R. E. and Todaro, G. J.: Multiple divergent copies of endogenous type C virogenes in mammalian cells. Nature 252: 170-173,1974. 16. Benveniste, R. E. and Todaro, G. J.: Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc. Natl. Acad. Sci. USA 71: 4513-4518,1974. 17. Benveniste, R. E., Sherr, C. J., Lieber, M. M., Callahan, R. and Todaro, G. J.: Evolution of primate type-C viral genes. In: Fundamental Aspects of N eoplasia. Gottlieb, A. A., Plescia, 0. J. and Bishop, D. H. L. (Eds.). SpringerVerlag, New York, 1975, pp. 29-53. 18. Todaro, G. J., Sherr, C. J., Benveniste, R. E., Lieber, M. M. and Melnick, J. L.: Type C viruses of baboons: Isolation from normal cell cultures. Cell 2: 55-61,1974. 19. Benveniste, R. E., Heinemann, R., Wilson, G. L., Callahan, R. and Todaro, G. J. : Detection of baboon type C viral sequences in various prima te tissues by molecular hybridization. J. Viral. 14: 56-67,1974. 20. Sherr, C. J., Benveniste, R. E. and Todaro, G. J.: Type C viral expression in primate tissues. Proc. Natl. Acad. Sci. USA 71: 3721-3725,1974. 21. Sherr, C. J. and Todaro, G. J.: Type C viral antigens in man. I. Antigens related to endogenous primate virus in human tumors. Proc. Natl. Acad. Sci. USA 71: 4703-4707,1974. 22. Benveniste, R. E. and Todaro, G. J.: Evolution of C-type viral genes: In heritance of exogenously acquired viral genes. Nature 252: 456-459, 1974. 23. Todaro, G. J., Benveniste, R. E., Callahan, R., Lieber, M. M. and Sherr, C. J.: Endogenous primate and feline type C viruses. Cold Spring Harbor Symp. Quant. BioI. 39: 1159-1168,1974. 24. Baluda, M. A. and Roy-Burman, P.: Partial characterization of RDl14 virus by DNA-RNA hybridization studies. Nature New BioI. 244: 59-62, 1973. 25. Neiman, P. E.: Measurement of RDl14 virus nucleotide sequences in feline cellular DNA. Nature New BioI. 244: 62-64,1973. 26. Benveniste, R. E., Sherr, C. J. and Todaro, G. J.: Evolution of type C viral genes: Origin of feline leukemia virus. Science 190: 886-888, 1975. 27. Benveniste, R. E. and Todaro, G. J.: Evolution of type C viral genes. III. Preservation of ancestral murine type C viral sequences in pig cellular DNA. Proc. Natl. Acad. Sci. USA 72: 4090-4094,1975. 28. Breese, S. S.: Virus-Iike particles occurring in cultures of stable pig kidney cell lines. Archiv Gesamte Virusforsch 30: 401-404,1970. 29. Strandström, H., Veijalainen, P., Moennig, V., Hunsmann, G., Schwarz, H. and Schäfer, W.: C-type particles produced by a permanent cell line from a leukemic pig. I. Origin and properties of the host cells and some evidence for the occurrence of C-type-like particles. Virology 57: 175-178,1974. 30. Todaro, G. J., Benveniste, R. E., Lieber, M. M. and Sherr, C. J.: Characterization of a type C virus released from the porcine cell line PK(15). Virology 58: 65-74,1974. 31. Lieber, M. M., Sherr, C. J., Benveniste, R. E. and Todaro, G. J.: Biologic and immunologic properties of porcine type C viruses. Virology 66: 616-619,1975. 32. Kawakami, T. G., Huff, S. D., Buckley, P. M., Dungworth, D. L., Snyder, S. P. and Gilden, R. V.: C-type virus associated with gibbon lymphosarcoma. Nature New BioI. 235: 170-171,1972. 33. Theilen, G. H., Gould, D., Fowler, M. and Dungworth, D. L.: C-type virus in tumor tissue of a woolly monkey (Lagothrix ssp.) with fibrosarcoma. I. Natl. Cancer lnst. 47: 881-889,1971. 34. Wolfe, L. G., Deinhardt, F., Theilen, G. H., Rabin, H., Kawakami, T. G. and Bustad, L. K. : Induction of tumors in marmoset monkeys by simian sarcoma virus, type I (Lagothrix): A preliminary report. I. Natl. Cancer lnst. 47: 1115-1120,1971. 35. Parks, W. P., Scolnick, E. M., Noon, M. C., Watson, C. J. and Kawakami, T. G.: Radioimmunoassay of mammalian type C polypeptides. IV. characterization of woolly monkey and gibbon viral antigens. lnt. I. Cancer 12: 129-137, 1973. 36. Kawakami, T. G., Buckley, P. M., McDowell, T. S. and DePaoli, A.: Antibodies to simian C-type virus antigen in sera of gibbons ( Hylobates sp.) Nature New BioI. 246: 105-107,1973. 37. Todaro, G. J., Lieber, M. M., Benveniste, R. E., Sherr, C. J., Gibbs, C. J. Jr., and Gajdusek, D. C. : Infectious primate type C viruses: Three isolates belonging to a new subgroup from the brains of normal gibbons. Virology 67: 335-343, 1975. 38. Scolnick, E. M., Parks, W., Kawakami, T., Kohne, D., Okabe, H., Gilden, R. and Hatanaka, M. : Primate and murine type C viral nucleic acid association kinetics: Analysis of model systems and natural tissues. I. Virol. 13: 363-369, 1974. 39. Kawakami, T. G. and Buckley, P. M.: Antigenic studies in gibbon type-C viruses. Transplantation Proc. 6: 193-196,1974. 40. Ben veniste, R. E. and T odaro, G. J. : Homology between type-C viruses of various species as determined by molecular hybridization. Proc. Natl. Acad. Sci. USA 70: 3316-3320, 1973. 41. Sherr, C. J., Fedele, L. A., Benveniste, R. E. and Todaro, G. J.: Interspecies antigenic determinants of the reverse transcriptases and p30 proteins of mammalian type C viruses. I. Virol. 15: 1440-1448,1975. 42. Lieber, M. M., Sherr, C. J., Todaro, G. J., Benveniste, R. E., Callahan, R. and Coon, H. G.: Isolation from the Asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc. Natl. Acad. Sci. USA 72: 2315-2319, 1975. 43. Todaro, G. J. and Gallo, R. C. : Immunological relationship of DNA polymerase from human acute leukaemia cells and primate and mouse leukaemia virus reverse transcriptase. Nature 244: 206-209, 1973. 44. Gallagher, R. E., Todaro, G. J., Smith, R. G., Livingston, D. M. and Gallo, R. C.: Relationship between RNA-directed DNA polymerase (reverse transcriptase) from human acute leukemic blood cells and primate type-C viruses. Proc. Natl. Acad. Sci. USA 71: 1309-1313, 1974. 45. Miller, N. R., Saxinger, W. C., Reitz, M. S., Gallagher, R. E., Wu, A. M., Gallo, R. C. and Gillespie, D. : Systematics of RNA tumor viruses and viruslike particles of human origin. Proc. Natl. Acad. Sci. USA 71: 3177-3181, 1974. 46. Mak, T. W., Kurtz, S., Manaster, J. and Housman, D.: Viral-related information in oncornavirus-like particles isolated from cultures of marrow cells from leukemic patients in relapse and remission. Proc. Natl. Acad. Sci. USA 72: 623-627,1975. 47. Sherr, C. J. and Todaro, G. J.: Primate type C virus p30 antigen in cells from humans with acute leukemia. Science 187: 855-857,1975. 48. Gallagher, R. E. and Gallo, R. C.: Type C RNA tumor virus isolated from cultured human acute myelogenous leukemia cells. Science 187: 350-353, 1975. 49. Panem, S., Prochownik, E. V., Reale, F. R. and Kirsten, W. H. : Isolation of type C virions from a normal human fibroblast strain. Science 189: 297-299, 1975. 50. Nooter, K., Aarssen, A. M., Bentvelzen, P., de Groot, F. G. and van Pelt, F. G.: Isolation of infectious C-type oncornavirus from human leukaemic bone marrow cells. Nature 256: 595-597,1975. 51. Gabelman, N., Waxman, S., Smith, W. and Douglas, S. D.: Appearance of C-type virus-like particles after co-cultivation of a human tumor-cell line with rat (XC) cells. lnt. I. Cancer 16: 355-369, 1975. 52. Teich, N., Weiss, R. A., Salahuddin, S. Z., Gallagher, R. E., Gillespie, D. H., Gallo, R. C. : Infective transmission and characterization of a C-type virus released by cultured human myeloid leukaemia cells. N ature 256: 551-555, 1975. 53. Gallagher, R. E., Salahuddin, S. Z., Hall, W. T., McCredie, K. B. and Gallo, R. C. : Growth and differentiation in culture of leukemic leukocytes from a patient with acute myelogenous leukemia and reidentification of a type-C virus. Proc. Natl. Acad. Sci. USA 72: 4137-4141,1975. 54. Strand, M. and August, J. T. : Type-C RNA virus gene expression in human tissue. I. Virol. 14: 1584-1596,1974. 55. Hardy, W. D. Jr., Old, L. J., Hess, P. W., Essex, M. and Cotter, S.: Hori zontal transmission of feline leukaemia virus. N ature 244: 266-269, 1973. 56. Olson, C., Miller, L. D., Miller, J. M. and Hoss, H. E. : Transmission of lymphosarcoma from cattle to sheep. I. Natl. Cancer Inst. 49: 1463-1468, 1972. 57. T odaro, G. J. : " Spon taneous " release of type C viruses from clonal lines of "spontaneously" transformed Balb/3T3 cells. Nature New Bioi. 240: 157-160, 1972. 58. Lieber, M. M. and Todaro, G. J.: Spontaneous and induced production of endogenous type-C RNA virus from a clonal line of spontaneously trans formed Balb/3T3. Int. I. Cancer 11: 616-627,1973. 59. Rapp, U. R., Nowinski, R. C., Reznikoff, C. A. and Heidelberger, C.: Endogenous oncornaviruses in chemically induced transformation. I. Trans formation independent of virus production. Virology 65: 392-409, 1975. 60. Barbieri, D., Belehradek, J. Jr., and Barski, G.: Decrease in tumor-producing capacity of mouse cell lines following infection with mouse leukemia viruses. Int. I. Cancer 7: 364-371, 1971. 61. Scolnick, E. M., Rands, E., Williams, D. and Parks, W. P.: Studies on the nucleic acid sequences of Kirsten sarcoma virus: A model for formation of a mammalian RNA-containing sarcoma virus. I. Virol. 12: 458-463, 1973. 62. Weiss, R. A., Mason, W. S. and Vogt, P. K.: Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology 52: 535-552, 1973. 63. Anderson, N. G.: Evolutionary significance of virus infection. Nature 227: 1346,1970. |