|
* This research was supported in part by Grants CAl6673 and CA13l48, awarded by the National Cancer Institute Soon after it was established that normal lymphoid development proceeds along two distinct pathways of differentiation, it was recognized that lymphoid malignancies affected cells of either T or B lineage, and not both [ I]. Studies conducted with animal models of lymphoblastic leukemias and lymphomas revealed that malignant T and B cells, like their normal counterparts, have their origin in central lymphoid tissues. The thymus is essential in the genesis of a variety of murine lymphoid malignancies that are induced by oncogenic viruses, ionizing irradiation, carcinogenic hydrocarbons, and hormones, or that arise spontaneously in AKR mice (reviewed [2]). Removal of the thymus prevents these lymphoid malignancies, and thymus transplants restore susceptibility [2-4]. This is due to an initial transformation of thymocytes with subsequent seeding or metastasis to peripheral tissues. On the other hand, the bursa of Fabricius is the source of malignant B cells in avian lymphoid leukosis [5,6]. Avian lymphoid leukosis was the first model of a virus-induced B-cell malignancy and several of its features are relevant to the analysis of human B-cell malignancies. This B-cell lymphoma can be induced by infection of embryos or newly hatched chicks with avian group A leukemia retroviruses [7, 8]. The virus infects many cell types, but it selectively transforms B cells [6. 9]. Moreover, the virus-induced transformation only occurs at a very early stage in B-cell differentiation within the in ductive bursal microenvironment. There are two distinctive phases in the evolution of this virus-induced malignancy of selected B-cell clones. First, one or more of the thousands of lymphoid follicles within the bursa exhibit lymphoblastic transformation. The transformed follicles are evident within 1-2 months after virus infection at hatching. The next phase usually occurs between 5 and 9 months of age, and involves widespread seeding and malignan t growth of B cells, most of which do not become mature plasma cells. Bursectomy or physiological bursal regression prior to this second stage will abort the fatal B-cell malignancy [5,6]. In the lymphoma cells, viral promoter sequences have been found to be integrated with a cellular onc gene called c-myc [ 10]. The activation of this transforming gene may be responsible for the initial transformation of lymphoid cells in the bursal follicles. However, the activated c-myc gene is unrelated to the transforming gene that has been detected by transfection with lymphoma DNA [ II]. Activation of the latter onc gene could be responsible for the second step in the evolution of a malignant B-cell clone. Another hypothesis is that antigen-induced growth of transformed B cells may playa significant role in the malignant lymphomatosis phase [9]. The retrovirus itself could serve as the stimulating antigen for B-cell clones with appropriate immunoglobulin receptors [ 12]. We hypothesized that human B-cell malignancies would also involve an initial transformation of B-cell clones within the inductive microenvironment and that antigens could influence the subsequent be havior of the affected clones. In this chapter we review the results of our studies on a spectrum of human B-cell malignancies within the context of normal B-cell differentiation. To identify the affected B cells we have used the immunoglobulins which they produce as clonal markers. Antibodies were prepared against immunoglobulin heavy and light chain isotypes, VH subgroups and idiotypes, and these were used to diagnose and analyze pre-B leukemias, B-cell leukemias, W aldenström 's macroglobulinemia, and multiple myelomas.
Cells of B lineage are unique in their expression of the immunoglobulin genes, and progression along this differentiation pathway can be discerned by determining which immunoglobulin genes are expressed. Immunoglobulin molecules consist of identical pairs of heavy and light chains. In the mouse, these are encoded by linked families of VH, DH, and lH genes located 5' to the heavy chain constant region ( C H) genes, the order of which is µ, delta, gamma 3, gamma l, gamma 2b , gamma 2a, Epsilon , and a on chromosome 12 [13-15]. The VL and lL genes are upstream from the chi and lamda light chain genes on chromosomes 6 and 16, respectively [14, 16, 17]. The corresponding H, chi , and lamda immunoglobulin gene families in human are located on chromosomes 14, 2, and 22 [18-21]. One of the first steps in differentiation is the assembly by chromosomal rearrangement of one each of the VH, DH, and lH genes and the transcription of the V -D-l set along with the Cµ gene [22, 23]. A cell expressing such µRN A is known as a pre- B cell, and at this stage few of the µ chains reach the cell surface [24- 29] .Next, one set of VL and lL genes is productively rearranged and a complete IgM molecule is expressed on the cell surface. This differentiation event marks the birth of an immature B-lymphocyte, and the point at which antigens may begin to influence the cell's behavior. The foregoing stages, stem cell-+ preB-+ immature B, occur initially within inductive microenvironments of the fetal liver and thereafter in the bone marrow. Sub sequent stages in B-cell differentiation entail changes in the expression of the CH genes, without alteration in expression of the H-DH-lH set or the light chain gene. Intermediate stages in B-lymphocyte differentiation are marked by the expression ofa variety of cell surface proteins involved in regulating migration, growth, and differentiation of B cells into terminally differentiated plasma cells with the associated shift from surface expression to secretion of immunoglobulin molecules. Immature B cells first express surface IgM and later coexpress IgD with the same VH-DH-lH and light chains. Some members within each B cell clone undergo a further switch, from IgM (and IgD) to IgG, IgA, or IgE [30], and all members of the clone will of course share the same antibody specificity and idiotype. Current views on heavy chain isotype switching mechanisms, sequence, and regulation are reviewed elsewhere [31]. The number of B-cell clones within an individual is very large, probably well over a million. Each expresses a unique antibody specificity and idiotypic pattern, but may share cross-reactive idiotypes with other clones [32].
Approximately 20% of all acute lymphocytic leukemias of childhood
can be recognized as pre-B leukemias by the presence of intracytoplasmic
µ chains, absence of surface immunoglobulin (Ig), characteristic
lymphoid morphology with lobulated nucleus and marrow cytoplasmic
rim, surface expression of B-cell differentiation antigens, and
the absence of T -cell and myelomonocytic antigen markers [33-36].
Another large segment of acute lymphocytic leukemias, perhaps 50%-60%,
can be recognized as "pre" pre- B cells by detecting rearrangements
of immunoglobulin heavy chain genes [28] and the expression of B-cell
surface antigens [37-39]. Other characteristic features are the
expression of HLA-DR, common ALL antigen, and terminal deoxynucleotidyl
transferase activity [33-36]. There is suggestive evidence that
the more differentiated µ-t- pre-B leukemias have a worse prognosis
than the µ -pre- B leukemias [40]. Neither follows the relentless
and rapid downhill course of the childhood B-cell leukemias, which
are featured by surface IgM expression. The target cell for the
oncogenic events appears to be an Ig- bone marrow precursor cell.
Even in the µ + pre- B leukemias, some members of the leukemic clone
do not express µ chains. More compelling evidence comes from the
study of individuals with chronic myelogenous leukemia. Analysis
of chromosomal markers (i.e., the Philadelphia chromosomal aberration
on chromosome 22 and the G6PD isoenzymes, or alleles, encoded on
the X chromosomes) has revealed that normal blood cells in these
patients are derived from the same pluripotent stem cell as the
myelogenous leukemia cells [41, 42]. More relevant to our theme
here are the patients who undergo conversion from chronic myelogenous
leukemia to acute lymphocytic leukemia of pre-B phenotype. Chromosomal
marker analysis indicates that both lines of malignant cells are
sequentially derived from the same multipotent stem cells [43-45].
The patterns of immunoglobulin gene expression in pre-B leukemia
clones are also informative. Most pre-B leukemia cells express µ
chains but no light chains, a finding that is consistent with the
asynchronous onset of heavy and light chain expression observed
in normal pre-B cells [46-50]. Unlike normal pre-B cells, however,
subpopulations of pre-B cells within the leukemic clones may express
heavy chain isotypes other than µ [33, 35]. In order to examine
further the heavy chain isotope switching in leukemic pre-B cells,
we have used monoclonal antibodies in immunofluorescence assays
to allow unambiguous assignment of the heavy chain isotopes expressed
by individual leukemic cells. Switching in leukemic pre-B clones
from II childhood leukemias invariably led to expression of Yl heavy
chains, and less often to expression of Y4 and a [51]. The observed
frequencies of isotype switches, µ to gamma l > gamma 4 » alfa and
the absence of a, gamma 2, gamma 3, and Epsilon, indicate a preferential
order for the switching process in leukemic pre-B cell clones. Since
these cells lack surface immunoglobulins, these data favor a stochastic
model for isotype switching rather than an antigen-induced switch
mechanism. So far the order of human CH genes on chromosome 14 [18]
has only been partially elucidated. Cµ and Co are thought to be
next in line 3' to the JH genes as is the case in mice [52]. C gamma
2 is 5' to C gamma4 [53, 54]; C gammal appears to be 5' to CY3 [54];
and CEpsilon genes are thought to be located 5' to the Calfa l and
Calfa 2 gene [55]. Although our observations would fit with a gene
order in man of µ, a, gamma l, gamma 3, gamma 2, gamma 4' Epsilon,
and alfa , the data indicate that the switch sequence cannot be
explained solely by the CH gene order. The results of two-color
immunofluorescence analysis indicated that individual pre-B cells
within the leukemic clones express as many as three or even four
heavy chain isotypes [51]. The stability of these phenotypic patterns
has not been examined yet, but the presence of multiple heavy chain
isotypes in individual pre-B cells might be explained by the hypothesis
of a preliminary switch mechanism involving a large primary transcript
of all the CH genes and differential RNA splicing [56, 57]. This
hypothesis does not, however, simplify the problem of ordered switching
in the leukemic pre-B cells. Another remarkable finding in our studies
was the expression of x light chains by almost all of the leukemic
pre-B cells exhibiting heavy chain switches. This preference for
x over lamda expression might be expected in view of evidence which
suggests that Vlamda-Jlamda gene rearrangement for expression with
Clamda regularly follow nonproductive rearrangements of x genes
on both chromosomes [58-60]. However, the consistent acquisition
of a productive x gene by switching pre-B cells is unprecedented
and suggests that these genetic events, occurring on chromosomes
14 and 2 [18-20], are coupled by a regulatory mechanism that remains
to be elucidated. Occasional clones of leukemic pre-B cells appear
to continue differentiation into B-Iymphocytes. One such example
is illustrated in Fig. 1. Approximately half of the leukemic cells
in this patient were µ+ pre-B cells and the other half IgM lamda
-bearing B-lymphocytes. This suggests that the transformation process
per se does not necessarily preclude continued differentiation beyond
the pre-B compartment, and this principle has been confirmed in
the following studies of B-cellleukemias and multiple myelomas 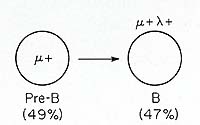
. C. B-Cell Leukemias B-cell leukemias are monoclonal Iymphoproliferative disorders marked
by the expression of surface immunoglobulin (reviewed in [61 ]).
Most of them express surface IgM, or IgM and IgD together. Less
frequently the leukemic B cells express IgG or IgA. B-cellleukemias
are closely related to B-celllymphomas; their distinction rests
primarily upon the predominant migration pattern of the involved
B-cell clone, i.e., lymphoid tissues versus circulation. The affected
B cells usually fail to differentiate into mature plasma cells,
although some malignant B-cell clones contain mature antibody-secreting
members, and many can be induced to differentiate into plasma cells
in vitro [62-64]. An ideal marker for malignant B cells is the idiotype
(Id) of the immunoglobulin that they express. Anti-Id antibodies
have been difficult to prepare for B-cell leukemias and lymphomas,
because they produce so little of their immunoglobulin product.
However, hybridoma technology now makes it feasible to make monoclonal
antibodies to the Id determinants expressed by malignant B-cell
clones. We have prepared monoclonal antibodies specific for the
Id determinants on leukemic B cells from selected patients, and
have used these anti-Id antibodies to trace the extent of clonal
involvement. Ninety percent of the circulating mononuclear cells
(18,000/mm³) in one such patient were small lymphocytes bearing
IgM lamda and IgD lamda molecules. Virtually all of these were reactive
with a monoclonal anti-Id antibody tailor made against her leukemic
B cells [65]. IgG and IgA B cells were very rare in this woman but,
of these, 40% and 25% were reactive with the same monoclonal anti-Id
antibody. This suggests that a few mem bers of the leukemic clone
have undergone heavy isotype switches, the frequency of which was
governed by ratelimiting feature of the switch process. Additional
information can be obtained by study of bone marrow in addition
to the blood cells in individuals with B-cell leukemia. This is
illustrated by our findings in an elderly man with acute lymphoblastic
leukemia cells that expressed surface IgAl chi molecules [66]. All
of his leukemic cells were reactive with one of a panel of four
monoclonal anti- VH subgroup antibodies [67]; a monoclonal anti-Id
antibody (WF) was prepared which reacted with all of the leukemic
IgA B cells and < 1% of normal B cells. N one of the plasma cells
found in this patient expressed the homologous idiotype, suggesting
that the leukemic B-Iymphocytes did not complete differentiation.
The expression of the WF idiotype by cells in the circulation was
restricted to the IgAl chi leukemic B cells; no T cells or IgM and
IgG B cells with the WF Id could be found. The picture was different
in the bone marrow of this patient (see Fig. 2). Here we found a
small subpopulation of IgM chi lymphoblasts that expressed the WF
idiotype and the same VH subgroup as the IgAlchi lymphoblasts. Pre-B
cells containing alfa, gamma , and µ chains of the same VH subgroup
were also present in the bone marrow. The lineal relationship between
the µ, gamma , and a pre-B cells was indicated by presence of both
µ and gamma , and of gamma and a together in some of these pre-B
cells. Light chain expression was not evident in these pre-B cells;
this precluded identification with the monoclonal anti-Id antibody,
because it recognized an idiotope formed by the heavy and light
chains combined. When the bone marrow sample was depleted of B-Iymphocytes
and placed in culture, IgAl chi B cells with the WF Id were generated.
These results suggest that this leukemic clone was transformed prior
to the heavy chain switch and before chi light chain expression.
The basis for the preferential expansion of the IgAl B-cell subpopulation
is unclear. These cells did not display translocations on chromosomes
14 or 2 as have been observed in IgM chi Burkitt's lymphomas [21,
68]. It is noteworthy, however, that examination of the DNA from
the IgAl leukemic cells failed to reveal deletion of all of the
gamma genes (1. Ellison, unpublished). Deletion of C H genes 5'
to the expressed CH gene on one or both chromosomes has been a consistent
feature in mouse myeloma cells, but this would appear to be the
first attempt to examine this switch event at the B-lymphocyte level.
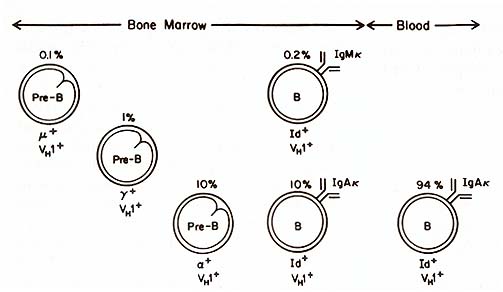
D. Plasma Cell Malignancies Multiple myeloma is a B-cell malignancy that has classically been thought to involve bone marrow plasma cells. This viewpoint has been modified by the demonstration of an increase in circulating B-Iymphocytes bearing the homologous idiotype. In two patients with IgA myelomas, we found expression of the homologous Id on IgM-I- / IgD-I- B-Iymphocytes as well as on IgA B-Iymphocyte precursors. Moreover, a few Id-l- cells of the µ-I- pre-B phenotype were identified in the bone marrow [69]. Similar observations were made in studies of a patient with an IgD myeloma [65]. Hence, we have proposed that even multiple myelomas have roots within the pre-B cell compartment, and the bone marrow predilection of the myeloma population may be due to its genesis from marrow stem cells. In a woman with Waldenström's macroglobulinemia, most of the circulating B cells had surface IgM with the homologous idiotype. In addition, 25% of her circulating IgA B cells expressed the same idiotype, suggesting that these cells belonged to the malignant clone as well [65]. The IgA -1 members of the clone were different from their sister IgM cells in that they apparently did not complete differentiation, i.e., we could find no IgA-I- Id-l- plasma cells and no serum IgA paraprotein. These results contrast with the extent of clonal involvement in another patient with a serum IgM paraprotein that had binding specificity for intermediate filaments (IMF). The involved clone also included IgGl, IgG3, IgAl, and IgA2 plasma cells which were identified by the homologous idiotype, VH subgroup, and antigen specificity (A. Landay, H. Kubagawa, and M. D. Cooper, unpublished). It is puzzling that different members of a malignant B clone can behave so differently with regard to proliferation and differentiation. It is of course possible that, like normal B cells, they are influenced differently by antigens and immunoregulatory T cells (e.g., see chapter by Gershon). The problem is usually complicated by the unknown antibody specificity of the immunoglobulins made by malignant B cells. In the above example, however, the antibodies were directed against a highly conserved determinant present on all IMF forms. Since it is on a basic cellular constituent, this antigen would be released with cell injury and hence available to stimulate immunocempetent cells. This could explain why individuals with hepatitis often produce high titers of antibodies to IMF [70]. The mere fact that 5%-10% of the IgM paraproteins in humans have IMF specificity [71] may in itself imply a role for antigens in the malignant behaviour of transformed B-cell clones.
These results are consistent with the idea that while B-cell malignancies
show great variability in their progression along normal differentiation
pathways, they undergo in common an initial transformation process
within the bone marrow environment (Fig. 3). An important corollary
of this hypothesis is that the events involved in initiation of
normal differentiation would also be engaged in the genesis of malignant
B-cell clones. Our data further suggest that the initial transformation
process is not always immediately followed by exaggerated overgrowth
of the B cells belonging to the affected clone. Lane and her co-workers
have obtained evidence in DNA transfection studies suggesting that
different transforming genes may be activated in neoplasms featuring
pre-B, B, or plasma cells [72]. Their results indicate that specific
transforming genes are activated in neoplasms corresponding to specific
stages of differentiation within the cell lineage. The hypothesis
that human B-cell malignancies involve the sequential activation
of at least 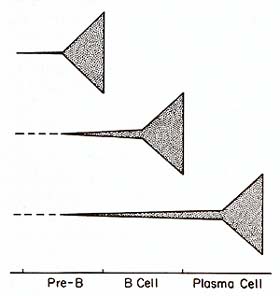
two transforming genes, as may be the case in the avian lymphoma
model [11], would easily accomodate both sets of observations. Still
to be explained is the great variability in the growth and differentiation
behavior of different members within neoplastic B-cell clones, and
why B-cell clones with certain antigen specificities are more frequently
involved. In view of these features, and the demonstration that
immunoregulatory T cells can modify growth and differentiation of
neoplastic B-cell clones [73, 74], it is still plausible that antigens
and T cells may be significant modifiers of human B-cell tumors.
It should be mentioned, however, that pre-B cell leukemias represent
a clear exception to the idea that antigens may influence growth
and differentiation of neoplastic B cells, and since the pre-B leukemia
cells lack the surface imm unoglobulin with which to see antigen,
they would not be expected to be clonally regulated by the usual
immunoregulatory controls. Acknowledgments We thank Mrs. Ann Brookshire for preparing this manuscript.
24. Cooper MD (1981) Pre-B cells: Normal and abnormal development. J Clin Immunol 1:81-89 25. Maki R, Kearney J, Paige C, Tonegawa S ( 1980) Immunoglobulin gene rearrangement in immature B cells. Science 209: 1366-1369 26. Perry RR, Kelley DE, Coleclough C, Kearney ]F (1981) Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci USA 78:247-251 27. Alt F, Rosenberg N, Lewis S, Thomas E, Baltimore D (1981) Organization and reorganization of immunoglobulin genes in A-MuL V -transformed cells: Rearrangement of heavy but not light chain genes. Cell 27:381-390 28. Korsmeyer SJ, Hieter RA, Ravetch JV, Poplack DO, Waldmann TA, Leder P (1981) Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B cells. Proc Natl Acad Sci USA 78:7096-7100 29. Paige C], Kincade PW, Ralph P (1981) Independent control of immunoglobulin heavy and light chain expression in a murine pre-B cell line. Nature 292: 631-633 30. Cooper MD, Keamey JF, Oathings WE, Lawton AR (1980) Effects of anti-Ig antibodies on the development and differentiation of E cells. lmmunol Rev 52:29-53 31. Marcli KE, Cooper MD (1982) New views of the immunoglobulin heavy-chain switch. Nature 298: 327-328 32. Berek C, Etlinger H, Julius M (eds) (1982) Idiotypes: Antigens on the Inside, Workshop at the Basel Institute for Immunology, November 19-20, 1981, F. Hoffman-La Roche & Co. Limited, Basel 33. Vogler LB, Crist WM, Bockman DE, Pearl ER, Lawton AR, Cooper MD (1978) Pre-B cell leukemia: A new phenotype of childhood lymphoblastic leukemia. N Engl J Med 298:872-878 34. Brouet JC, Preud'homme JL, Penit C, Valensi F, Rouget P, Seligmann M (1979) Acute lymphoblastic leukemia with pre-B cell characteristics. Blood 54: 269-273 35. Vogler LB, Preud'homme JL, Seligmann M, Gathings WE, Crist WM, Cooper MD, Boilum FJ (1981) Diversity of immunoglobulin expression in leukaemic cells resembling B-lymphocyte precursors. Nature 290: 339-341 36. Greaves M, Verbi W, Vogler L, Cooper M, Ellis R, Ganeshagura K, Hoffbrand V, Janossy G, Bollum F J (1979) Antigenic and enzymatic phenotypes of the pre-B subclass of acute lymphoblastic leukaemia. Leukemia Res 3:353-362 37. Balch CM, Dougherty PA, Vogler LB, Ades EW, Ferrone S (1979) Anew B cell differentiation antigen (BOA) on normal and leukemic human B lymphocytes that is distinct from known DR (Ia-Iike) antigens. J Immunol121:2322-2328 38. Abramson CS, Kersey JH, LeBien TW (1981) A monoclonal antibody (BA-I) reactive with cells of human B lymphocyte lineage. J Immunoll26: 83-88 39. Nadler LM, Ritz J, Hardy R, Pesando JM, Schlossman SF (1981) A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin In vest 67: 134-140 40. Pullen OJ, Crist WM, Falletta JM, Boyett JM, Roper MA, Dowell B, van Eys J, Humphrey GB, Head D, Brock BL, Blackstock R, Metzgar RS, Cooper MD (to be published) ALinC 13 classification protocol for acute lymphocytic leukemia: Characterization of immunologic phenotypes and correlation with treatment results. In: Proceedings of the St. Jude Leukemia Symposium 41. Fialkow PJ, Jacobson RJ, Papayannopoulou T (1977) Chronic myelocytic leukemia: Clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med 63: 125-130 42. Fialkow PJ, Denman AM, Jacobson RJ, Lowenthal MN ( 1978) Chronic myelocytic leukemia: Origin of some lymphocytes from leukemic stem cells. J Clin Invest 62:815-823 43. Vogler LB, Crist WM, Vinson PC, Sarrif A, Brattain MG, Coleman MS ( 1979) Philadelphia-chromosome-positive pre-B cell leukemia presenting as blast crisis of chronic myelogenous leukemia. Blood 54: 1164-1169 44. Greaves MF, Verbi W, Reeves BR, Hoffbrand A V, Drysdale HC, Jones L, Sacker LS, Samaratunga I (1979) "Pre-B" phenotypes in blast crisis of Ph1 positive CML: Evidence for a pluripotential stem cell "target". Leukemia Res 3: 181-191 45. LeBien TW, Hozier J, Minowada J, Kersey JH (1979) Origin of chronic myelocytic leukemia in a precursor of pre-B lymphocytes. N Engl J Med 301: 144-147 46. Burrows PD, LeJeune M, Kearney JF (1979) Evidence that mouse pre-B cells synthesise µ heavy chains but no light chains. Nature 280:838-841 47. Levitt D, Cooper MD (1980) Mouse pre-B cells synthesize and secrete µ heavy chains but not light chains. Cell 19:617-625 48. Gathings WE, Mage RG, Cooper MD, Young-Cooper GO (1982) A subpopulation of small pre-B cells in rabbit bone marrow expresses light chains and exhibits allelic exclusion of b locus allotype. Eur J Immunol 12:76-81 49. Kamps WA, Cooper MD (1982) Microenvironmental studies of pre-B and B cell development in human and mouse fetuses. J ImmunoI129:526-531 50. Kubagawa H, Gathings WE, Levitt D, Kearney JF, Cooper MD ( 1982) Imm unoglobulin isotype expression of normal pre-B cells as determined by immunofluorescence. J Clin ImmunoI2:264-269 51. Kubagawa H, Mayumi M, Crist WM, Cooper MD (to be published) Immunoglobulin heavy chain switching in pre-B leukemias. Nature 52. Ravetch JV, Siebenlist U, Korsmeyer S, Waldmann T, Leder P (1981) Structure of the human immunoglobulin µ locus: Characterization of em bryonic and rearranged J and D genes. Cell 27:583-591 53. Ellison J, Hood L ( 1982) Linkage and sequence homology of two human immunoglobulin gamma heavy chain constant region genes. Proc Natl Acad Sci USA 79:1984-1988 54. Takahaskhi N, Ueda S, Obata M, Nikaido T, Nakai S, Honjo T (1982) Structure of human immunoglobulin gamma genes: Implications for evolution of a gene family. Cell 29:671-679 55. Max EE, Battey J, Ney R, Kirsch IR, Leder P (1982) Duplication and deletion in the human immunoglobulin Epsilon genes. Cell 29:691-699 56. Alt FW, Rosenberg N, Casanova RJ, Tho mas E, Baltimore D (1982) Immunoglobulin heavy-chain expression and class switching in a murine leukemia cell line. Nature 296:325-331 57. Yaoita Y, Ktimagai Y, Oktimura K, Honjo T (1982) Expression of lymphocyte surface IgE does not require switch recombination. Nature 297: 697 -699 58. Alt FW, Enea V, Bothwell ALM, Baltimore D (1980) Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell 21 : 1-12 59. Hieter PA, Korsmeyer SJ, Waldmann TA, Leder P (1981) Human immunoglobulin chi light chain genes are deleted or rearranged in lamda -prodticing B cells. Nature 290: 368-372 60. Coleclotigh C, Perry RP, Karjalainen K, Weigert M (1981) Aberrant rearrangments contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature 290:371-378 61. Godal T, Ftinderud S ( 1982) Human B cell neoplasms in relation to normal B cell differentiation and maturation processes. Adv Cancer Res 36:211-255 62. Fu SM, Chiorazzi N, Ktinkel HG, Halper JP, Harris SR (1978) Induction of in vitro differentiation and immunoglobulin synthesis ofhtiman leukemic B lymphocytes. J Exp Med 148:1570-1578 63. Robert KH ( 1979) Induction of monoclonal antibody synthesis in malignant human B cells by polyclonal B cell activators: Relationship between B cell subsets and prognosis. Immtinol Rev 48: 123-143 64. Saiki 0, Kishimoto T, Kuritani T, Mtiragtichi A, Yamamura Y (1980) In vitro induction of IgM secretion and switching to IgG production in human B leukemic cells with the help of T cells. J Immtinol 124:2609-2614 65. Ktibagawa H, Maytimi M, Gathings WE, Kearney JF, Cooper MD (to be published) Extent of clonal involvement in B cell malignancies. In: Murphy S, Gilbert J (eds) Leukemia research: Advances in cell biology and treatment. Elsevier/North-Holland, New York 66. Maytimi M, Ktibagawa H, Omura GA, Gathings WE, Keamey JF, Cooper MD (1982) Studies on the clonal origin ofhtiman B cell leukemia using monoclonal anti-idiotype antibodies. J ImmtinoI129:904-910 67. Ktibagawa H, Maytimi M, Keamey JF, Cooper MD (1982) Immunoglobulin VH determinants defined by monoclonal antibodies. J Exp Med 156: 1010-1024 68. Klein G ( 1981) The role of gene dosage and genetic transposition in carcinogenesis. Nature294:313-318 69. Ktibagawa H, Vogler LB, Capra JD, Conrad ME, Lawton, Cooper MD (1979) Studies on the clonal origin of multiple myeloma: Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B cell differentiation. J Exp Med 150: 792-807 70. Mackay IR, Toh BH, Pederson JS (1981) A utoan tibodies to cytoskeletal filaments, actin and intermediate filaments, segregate with different types of chronic hepatitis. In: The Walter and Eliza Hall Institute of Medical Research. Ann ual Review 1980-81 , pp 96-97 71. Dellagi K, Brotiet JC, Perreati J, Patilin D (1982) Human monoclonal IgM with autoantibody activity against intermediate filaments. Proc Natl Acad Sci USA 79:446-450 |