G.F. Pluzhnikova 1, O.V. Plutalov 3, U.A. Berlin 3, and E.I. Scwartz 2 Hämatol. Bluttransf. Vol 35 |
|
1 The N. N. Petrov Research Institute of On cology,
USSR Ministry of Health, Leningrad, USSR.
It is widely accepted that tumor development and progression are due to illegitimate activation of cellular oncogenes by point mutation, retroviral insertion, chromosomal translocation and amplification or deletion of the gene (1-3]. Nonrandom deletions of chromosomal regions 13q14 and 11p13 have been detected in retinoblastoma (1] and Wilm's tumor (2, 3]. It has been proposed that these rare childhood cancers result from the deletion of dominant-acting genes, permitting the expression of tumorigenic recessive alleles (1]. Moreover, restriction fragment length polymorphism (RFLP) analysis has demonstrated loss of H-rasI oncogene allele (chromosome 11 p 15) in primary bladder, breast, ovarian, and lung carcinomas (4-7]. On the other hand, another important mechanism of activation of ras oncogene (including H-rasI) have been shown in 10 15 % of certain types o f human tumors, which involved a point mutation, causing an alteration at amino acid positions 12, 13 or 61 of the ras gene product p21 ras (8]. The study discusses the possible supressive action of the wild-type H-rasI allele on the mutant one in breast cancer.
The RFLP of H-rasI oncogene was analyzed in 76 primary breast carcinomas
as described (7]. H-rasI sequence spanning 145 base pairs across
codon 12 was amplified in vitro by Thermus thermophyIus DNA polymerase
(9]. Subsequent MspI digestion allowed us to detect the mutation
in "hot spot" due to the loss of the restriction site for Msplin
the case of substitution in the 12th codon of H-rasI (10]. 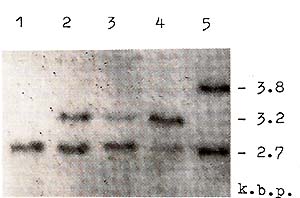 Fig. 1. Deletions of one of H-rasI allele in breast carcinomas (BC), identified by means of PvuII restriction of DNA samples, Southern blotting and hybridization with 6,6-fragment of pEl [11]. Samples of DNA were derived from (1) BC12 -genotype A1/A1; (2) leukocytes of BC9 -constitutive genotype A1/A2; (3) BC9 -A 1/ A2, deletion of A2 allele; (4) BC5 -A1/A2, deletion of A1 allele; and (5) BC31 -genotype A 1 /A3. Slight hybridization signals at the place of lost A2 (3) and A 1 (4) alleles are due to the contamination of the tumors by normal cells. In BC 107 and BC 109 the same deletions were detected as in BC9 (3) 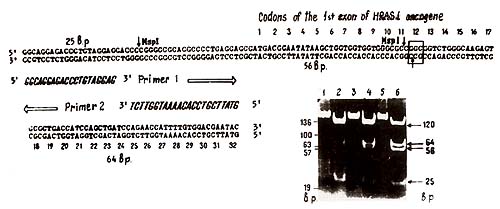 Fig. 2. Detection of H -rasI mutation in codon 12. The 145 base pair (bp) DNA sequence across 12th codon (in theframe) was amplified in vitro using the oligonucleotide primers (large open arrows indicate the 5' 3' orientation of the primers [9]. MspI digestion of the amplified sequences result in three fragments (25, 56, and 64 bp) in the case of wild-type allele, and two fragments (25 and 120 bp) in the case of substitution in codon 12 that altered msp site of restriction (vertical arrows). The photograph demonstrates the products of amplification of BC5 (I), BC9 (3), and BC 107, BC 109 (not shown) DNA samples and MspI restriction (3, 4, and 6, respectively). Arrows on the right of the photograph indicate bands corresponding to mutant and wild-type alleles. Alu fragments of pBR322 DNA (standard) are pointed out on the left of the photo 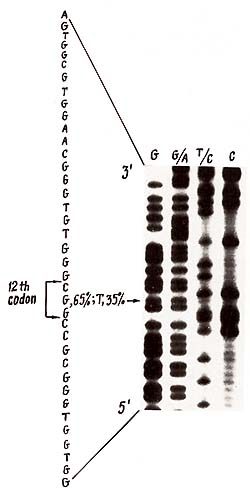
The loss of intact H -rasI allele and consequently its product -p21ras -normally involved in transport of mitogenic signal in the cell, might potentiate transforming activity of the oncoprotein, coded by the mutant allele. The deletion of wild-type allele of H-rasI oncogene is likely to unmask the mutant one. Nevertheless it is possible that another cellular constraint of growth is present on chromosome llp13-p15, and that the loss of this suppressor locus leads to activation of normally repressed class of genes.
|