|
1 Institute of Experimental Pathology and Therapy, USSR Academy of Medical Sciences, Sukhumi, USSR Tremendous progress has been achieved in cancer research in the
past decade. It is widely accepted that the major breakthrough is
in the field of oncogenes. However, this direction of cancer research
has not yet contributed significantly to an understanding of the
etiology of human leukemia. On the other hand, in the past few years
we have witnessed a renaissance in the field which I would call
"classical virology. " It began with the isolation by Gallo and
coworkers [1] of the first human retrovirus, HTL V -I, implicated
in the etiology of adult T -cellleukemia/lymphoma [2, 3]. Here I
will describe some aspects of a nonhuman primate model of human
lymphoma which we and our collaborators in many countries have been
working with for almost 20 years. This model was studied within
the framework of the above-mentioned classical virological approach,
and we have found many interesting and unique parallels with the
human system. The story began almost 20 years ago, when, with the
aim of isolating a hypothetical human leukemia virus, we inoculated
some baboons of our colony with pooled fresh blood of leukemia patients.
We were too optimistic and expected that in a few months we would
have a human leukemia virus in our hands. But after a year the inoculated
animals did not show any symptoms of leukemia. Our optimism sharply
decreased, and due to some difficulties in keeping these monkeys
in strict isolation we transferred them to open-air compounds where
they had close contact with untreated animals. To our surprise,
approximately 2 years after the inoculation, some of the treated
animals developed malignant lymphoma (Fig.l), and, totally unexpectedly,
some of their untreated neighbors developed the same disease. It
should be noted that our monkey colony was established in 1927 and
we now have the eleventh generation of baboons born in captivity.
However, we had never seen lymphoma in this simian species before
the introduction of human leukemic material. Quite frankly, we still
do not know what role this human leukemic material played in the
development of malignant lymphoma in our baboons. Nevertheless,
it appeared, and with these cases an outbreak of malignant lymphoma
started; to date almost 270 baboons have died of this disease. The
mortality of the disease fluctuates, being around 1.8%-2% per year
in a susceptible agegroup, which consists of animals over 3-4 years
of age (see Table 1). There are now 1200 baboons in this age-group
in our baboon stock, and this means that every year we register
up to 20 animals as dead or killed because of malignant lymphoma
[4, 5]. Our first impression was that the disease was monomorphic.
But later on, after thorough morphological investigations, we came
to the conclusion that there were many morphological variants of
the baboon malignant lymphoma. In some cases we observed Hodgkin-type
lymphomas; the rest of the cases were non-Hodgkin types including
lymphoblastic, prolymphocytic, immu noblastic, and some other variants
(Figs. 2-4). 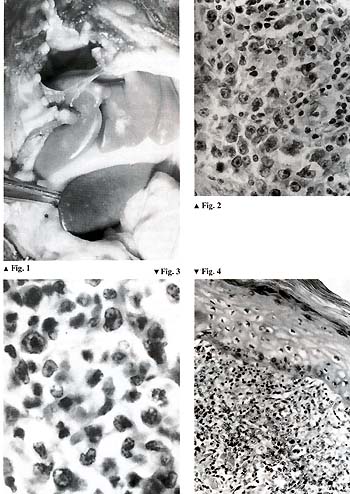
Table I. Lymphoma mortality among the
baboons of the Sukhumi monkey colony 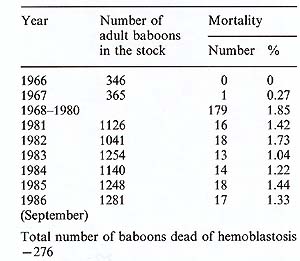
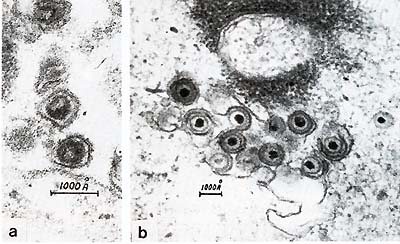 Fig.5. a Type-C retrovirus; b herpesvirus The disease could be transmitted with the cell-containing materials, and we started our attempts to isolate the viruses which might be responsible for the development of the disease. We isolated two types of oncogenic viruses. The first was a B-lymphotropic EBV -like herpesvirus which we called Herpesvirus papio (HVP) [5,6]. This virus is very closely related to EBV [7]. It immortalizes primate B-lymphocytes in vitro and has antigens cross-reacting with corresponding EBV antigens [8]. The genome structure of both viruses is the same, and the overall homology of EBV and HVP DNAs approximates 40% [7]. The second virus is a C-type retrovirus which belongs to the HTL V -I family [9, 10] . It is closely related to HT L V -I but there are some differences [11, 12] (Fig.5). Although we did not compare baboon HTLV-I-like virus with various simian isolates called STLV-I, we have many reasons to suspect a close relationship between baboon isolate and other STL V -I, as well as some differences. Serological studies have revealed that infection with both viruses is quite common in our high-lymphoma-risk stock [10, 12-14]. The prevalence of infection with both viruses increases with age, which indicates the horizontal transmission of these viruses within the colony. HVP virus is more contagious than STLV-I. The data presented illustrate the dynamics of the infections in high-lymphoma-risk stock. It should be noted that similar studies of ours with wild animals have shown some of them also to be infected with both HVP and STLV-I. But in this case the prevalence of infection was much lower, especially in the case of STLV-I. The level of antibodies against both viruses increases in the prelymphoma period and as a rule decreases after lymphoma development. HVP-specific DNA has been found in lymphomatous spleen tissues and some normal baboon tissues [15]. We have also demonstrated the presence of integrated STL V -I provirus in the D N A of malignant lymph nodes. The integration was monoclonal, and in some cases we found several integrated proviruses, including defective ones [12]. All these baboon lymphomas occurred in our main stock, which we called a high-lymphoma-risk stock. As controls we have another stock composed of animals imported directly from the wilderness, that have never had contact with the high-risk stock animals. This control stock numbers approximately 600 animals, a over a period of 15 years we have observed no cases of malignantlymphoma. Thus, the material presented here shows great similarities between baboon lymphomas and those in human beings. The discovery of the two viruses HVP and STL V -I in baboon malignancy, with integration of STL V -I provirus into the DNA of lymphomatous tissue, and the characteristic dynamics of antibody titers with their elevation in the prelymphoma period make it possible to conclude that baboon malignant lymphoma is associated with DNA and RNA oncogenic viruses.
|