|
1 Dept. Oncology and Hematology, BMT-Unit,
2 Dept. of TransfusionMedicine.
3 Dept. Pediactric Hematology, University Hospital Eppendorf, Hamburg,
Germany
Introduction
The hematopoietic growth factors G-CSF and GM-CSF are able to stimulate
granulopoiesis after autologous transplantation of bone marrow or
blood stem cells (PBSC) and can also mobilize peripheral blood progenitor
cells [1, 2, 3, 4]. Rapid recovery of platelets has been observed
following addition of G-CSF mobilized PBSC to autologous bone marrow
transplants [3]. The kinetics of engraftment are mainly dependent
on the number and qualitiy of progenitor/stem cells in the graft
[5, 6, 7, 8]. We have studied the impact of higher doses of G-CSF
(Filgrastim) on the yield of CD34 positive cells, CFU-GM and mononucelar
cells in leukapheresis products in patients with Non Hodgkin Lymphomas,
Hodgkins disease, testicular cancer, AML and neuroblastoma as well
as in normal donors for allogeneic progenitor cell transplantation.
We report our experience with mobilization of GCSF in two different
doses: 10 µg/kg per day versus 24 (2 x 12) µg/kg per day for stem
cell collection, separation and transplantation.
Materials and methods
Mobilization: G-CSF (Filgrastim) was administered subcutaneously
for 5- 6 days at a daily dose of 10 µg/kg (group A) or 2x12 µg/kg
(group B) to 45 patients. Beyond this a total of 18 mobilizations
followed by 1 to 4 leukapheresis procedures, was carried out in
normal donors. 15 transplants, involving mobilized progenitor cells
either alone or in conjunction with bone marrow, were carried out.
The characteristics of the autologous patients are shown in table
1.
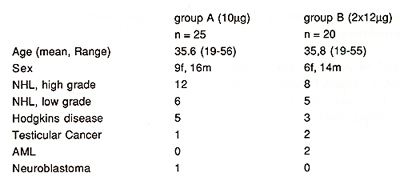
Table 1. Patient characteristics
15 patients with the following diagnosis: AML: 4, ALL: 5, Multiple
Myeloma: 1, Severe Aplastic Anaemia: 3, CML blastic crisis: 1, Myeloproliferative
Syndromes 1 received an allogeneic stem cell transplant. The donor-recipient
relationships were the following: matched siblings: 8, one mismatch:
2, parent to child: 3, child to parent: 1, matched unrelated donor:
1. Conditioning regimens: without conditioning as a rescue graft
for rescue from insufficient bone marrow function: 5; TBICyclophosphamide-VP16:
5; TBI-Cyclophosphamide: 1, Busulfan, Cyclophosphamid +- Etoposid:
4.
Colony forming assay: Colony forming units granulocyte-monocyte
(CFU-GM) were assayed in Iscove's methylcellulose. Colonies were
enumerated after 14 days of culture in 37° C, 5 % CO2 and 100 %
humidity by using an inverted phase microscope (9).
Flow cytometric analysis: Surface antigen expression was evaluated
using the monoclonal antibody HPCA-2, directly conjugated to FITC
(Becton-Dickinson). Analysis was performed on a FACScan (Becton-Dickinson)
using Lysis II research software [5,10,11].
Statistics: Data are shown as median (range), statistical significance
was determined by the Student's t-test.
Results
Autologous: The percentage of CD34+ mononuclear cells (MNC) in
the first leukapheresis product increased significantly (p=0.024)
from 0.68 (0.03-1.96) (group A) to 1.23 (0.4-4.28) (group B) (Fig.
1 ).
Fig.1 Leukapheresis products 10µg
vs. 2x12µg G-CSF
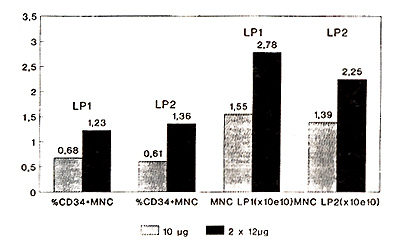
Statistically significant differences were also found with respect
to total CD34+ MNC per leukapheresis product (LP1): 10.08x10high
7 (0.4-44.0) (group A) versus 42.51 x 10 high 7 (3.46-86.3) (group
B) (p=0.012) (Fig. 2, Tabl. 2).
Fig. 2 Leukapheresis products 10µg vs.
2x12µg G-CSF
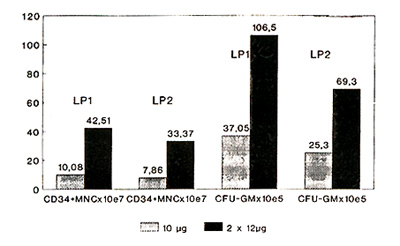
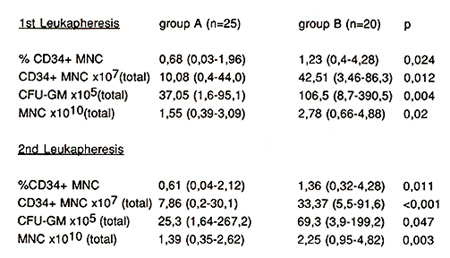
Table 2. Graft Data
CFU-GM increased from 37.05 (1.6- 95.1) x 105 (group A) to 106.5
(8.7- 390.5) (group B) (p = 0.004). These results confirm, that
higher doses of G-CSF can significantly increase the number of CD34
positive cells and CFU-GM in leukapheresis products.
Allogeneic: An average of 3 leukapheresis (range 1 to 4) were carried
out, which yielded 13 x 10 high8 (range 2.9 -29) mononuclear cells/kg
BW of the recipient, 5 x 10 high 6 CD34 positive cells/kg BW of
recipient (range 1.2- 39), CFC 3 x 10 high 5 /kg BW (0,88 60,4).
The mobilization with G-CSF was generally well tolerated with the
exception of moderate bone pain in 2/3 of the donors.
Engraftment in 9 evaluable patients revealed neutrophil engraftment
of more than 0,5/nl by day 14 (range 9 -22), neutrophils more than
1.0/nl day 15 (range 10- 24), thrombocytes more than 20/nl day 16
(range 8 -32), more than 50/nl 24 (range 13 to more than 100 days).
GvH grade 0 -1: 3 patients, grade 2: 4 patients, grade 4: 2 patients
and moderate chronic GvH in 1 patient. Total of 12 of the 15 patients
are alive in continuous complete remission, 3 have died of GvH and
infection (aspergillus 1, candida crusei 1 ).
Discussion
We could show that the mobilization with a higher than usual dosis
of G-CSF is well tolerated in normal donors as well as in patients
and yields grafts which allow engraftment after myeloablative therapy.
GvH occured in 2/3 of evaluable patients and led in conjunction
with infection to death in 3 patients. 1 patient received an allogeneic
graft from a matched unrelated donor, showed engraftment but unfortunately
died of a preexisting fungus disease.
Based on our experience in the autologous setting, where we found
statistically significant differences in the number of CD34+ cells
and CFU-GM in patients mobilized with 10 µg/kg G-CSF compared with
24 µg/kg and based on the observation that the side effects in both
dose levels are comparable, we would recommend 24 µg/kg BW G-CSF
per day, given in 2 divided doses or continuously intravenous infusion
for mobilization of normal donors.
References
1. Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L,
Griffin JD: Granulocyte-macrophage colony stimulating factor expands
the circulating haemopoietic progenitor cell compartment in man.
Lancet 1: 1194, 1988
2. Dührsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf
D: Effects of recombinant human granulocyte colony-stimulating factor
on hematopoietic progenitor cells in cancer patients. Blood 72:
2074, 1988
3. Sheridan WP, Begley CG, Jutner CA, Szer J, To LB, Maher D, McGrath
KM, et al. : Effect of peripheral-blood progenitor cells mobilised
by filgrastim (GCSF) on platelet recovery after high-dose chemotherapy.
Lancet 339: 640, 1992
4. Gianni AM, Siena S, Bregni M, Tarella C, Stern AC, Pileri A,
Bonnadonna G: Granulocyte-macrophage colony-stimulating factor to
harvest circulating haemopoietic stem cells for autotransplantation.
Lancet 2: 580, 1989
5. Siena S, Bregni M, Brando B, Belli N, Ravagnani F, Gandola L,
et al.: Flow cytometry for clinical estimation of circulating hematopoietic
progenitors for autologous transplantation in cancer patients. Blood
77: 400, 1991
6. Weaver CH, Buckner CD, Longin F, Appelbaum FR, Rowley S, Lilleby
K, et al. : Syngenic transplantation with peripheral blood mononucelar
cells collected after administration of recombinant human granulocyte
colonystimulating factor. Blood 82: 1981, 1993
7. Chao NJ, Schriber JR, Grimes K, Long GD, Negrin RS, Raimondi
CM, et al.: Granulocyte colony-stimulating factor "mobilized" peripheral
blood progenitor cells accelerate granulocyte and platelet recovery
after highdose chemotherapy. Blood 81: 2031, 1993
8. Bensinger W, Singer J, Appelbaum F, Lilleby K, Longin K, Rowley
S, et al.: Autologous transplantation with peripheral blood mononuclear
cells collected after administration of recombinant granulocyte
stimulating factor. Blood 81: 3158, 1993
9. Messner HA, Curtis JE, Minden MD, Tritchler D, Lockwood G, Takahashi
T, Lepine J, Jamal N, Tweeddale M, Wandl U: Clonogenic hemopoietic
precursors in bone marrow transplantation. Blood 70: 1425, 1987
10. Berenson RJ, Andrews RG, Bensinger WI, et al.: Selection of
CD34+ marrow cells for autologous marrow transplantation. Autologous
Bone Marrow Transplantation: Proceedings of the 4th International
Symposium, Dicke KA, Spitzer G, Jagannath S, et al. (eds.). Houston,
Univ. of Texas, 1989,55
11 . Shpall EJ, Jones RB, Bearman SI, Franklin WA, Archer PG, Curiel
T, Bitter M, et. al.: Transplantation of enriched CD34-positive
autologous marrow into breast cancer patients following high-dose
chemotherapy: influence of CD34-positive peripheral-blood progenitors
and growth factors on engraftment. Journal of Clinical Oncology
12: 1994, 28
|



