|
1 Johns Hopkins Oncology Center, 600 North Wolfe
Street, Baltimore, MD 21205, USA
A. Introduction
Allogeneic bone marrow transplantation in the treatment of acute
leukemia has shown remarkable therapeutic progress in recent years.
Long-term remissions and possible cure rates of 50% or higher have
been obtained by a number of centers in acute nonlymphocytic leukemia
(ANL), particularily when patients were transplanted in their first
remission [ 1-9]. Most reported series of allogeneic marrow transplantation
in acute lymphocytic leukemia (ALL) performed in the second remission
have shown long-term disease-free survival of 20%30%. Data for patients
in their third and subsequent remissions are less good [10-14].
The recent report from the Memorial Sloan Kettering group [15] and
the Baltimore group [16] has shown a therapeutic improvement over
the previously reported series of ALL transplanted in their second
remission. In the present communication, we wish to update our results
of allogeneic marrow transplantation in patients with ANL and ALL
who received marrow grafts from genotypically HLA-identical siblings.
B. Material and Methods
I. Informed
Consent All protocols were reviewed and approved by The Johns Hopkins
University Institutional Review Board.
II. Patient Selection
To be eligible for these studies, patients had to have a diagnosis
of ANL or ALL confirmed by examination of a marrow aspirate. In
addition, for ANL, they had to have a negative history for central
nervous system leukemia and, for both ANL and ALL, a spinal fluid
free of leukemic cells on cytocentrifuge examination at admission.
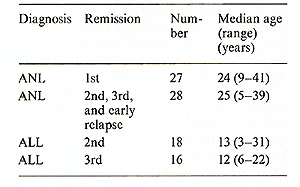
All data were analyzed as of 15 April 1984. A total of 27 patients
with ANL were transplanted in their first remission and 28 patients
in their subsequent remissions and early relapse; 18 patients with
ALL were transplanted to the second remission and 16 in their third
remission. The characteristics of each patient group are noted in
Table 1.
III. Marrow Grafts
Marrow aspiration was performed under general anesthesia. The technical
aspects of the marrow collection and administration were as described
previously [ 17].
Table I. Characteristics of patients with
ANL and ALL
IV. Preparation for Engraftment
Patients with ANL were prepared with oral busulfan (BU) given in
divided doses over a 4-da y period for a total dose of 16 mg/kg.
This was followed by cyclophosphamide (cY) given intravenously (i.v.)
at a dose of 50 mg/kg for four consecutive daily doses. Patients
with ALL were prepared with cy given i.v. at a dose of 50 mg/kg
for four consecutive daily doses followed by low dose rate total
body irradiation (TBI) of 300 rad/day for four consecutive daily
doses (lungs shielded for the third dose ). All patients received
one intrathecal injection of methotrexate (10 mg/m², but not more
than a total of 12 mg) before cytoreductive therapy.
V. Treatment After Marrow Grafting
Patients were given cy or cyclosporine prophylactically to prevent
graft-versushost disease. Prophylaxis for central nervous system
leukemia was given 50-80 days after marrow transplantation as five
intrathecal doses of methotrexate (10 mg/ m²) over 10-14 days.
C. Results
Analysis by Kaplan-Meier plots for patients with ANL revealed
an actuarial 3-year disease-free survival and median duration of
living survivors (range) for patients transplanted in the first
remission of 44% and 33.4 months (2.4-61.3 months) respectively.
Similar analysis of patients transplanted in second and subsequent
remission and early relapse revealed an actuarial 3-year disease-free
survival of 43%. The median survival for the survivors was 15.7
months with a range of 4.9-46.5 months. The 3-year probability of
diseasefree survival (for both groups of ANL patients combined)
for those aged 20 years or younger and older patients was 61% and
35%, respectively. There was only one leukemic relapse in this entire
series. This occurred I year after transplantation in a 36year-old
male transplanted in his third remission. Of 18 patients with ALL
transplanted in their second remission, 9 survive in continuous
remission from 1.2 to 49 months (median 19.2 months). The probability
of a 2-year disease-free survival is 48%. There have been no relapses
in this group. Of 16 patients (6 in continuous remission) with ALL
transplanted in their third remission, 8 survive for 2.3-46.8 months
(median 22.3 months) with a projected 2-year survival of 46%. Six
relapses were seen. The projected 2-year probability of remission
was 44%. The causes of deaths in both the ANL and ALL series of
patients were similar. Some 80% of the deaths were related to graft-versus-host
disease and viral infections.
D. Discussion
Our initial series of patients transplanted for ANL following preparative
treatment with BU and cy have been previously reported [8]. The
present extension of that study with additional time and more patient
entry continues to show promise. In particular, the very uncommon
relapses ( I of 55 patients) suggests that this regimen may well
have a more profound antileukemic effect than other reported treatments.
Other possible practical or future advantages for this preparative
treatment have been noted previously. The studies in ALL are not
quite so advanced, but already it appears that the transplantation
of patients with ALL following the CY- TBI protocol outlined here
results in a therapeutic response better than most reported series
and at the moment is at least similar to the Memorial-Sloan Kettering
experience using hyperfractionated TBI followed by cy [ 15]. Because
of the high relapse rate of ALL patients in the third remission,
we are currently preparing patients for transplantation with BU
and cy as outlined for ANL. Graft-versus-host disease and viral
infections continue to be a major cause of death. A number of laboratories
in transplant centers are intensively investigating approaches to
the prevention and treatment of these complications. Some of these
studies already show considerable promise. It is reasonable to assume
therefore, that within the next few years disease-free survivals
following allogeneic marrow transplantation may increase by 20%-30%.
Acknowledgements. This work was supported by PHS Grants CA-15396
and CAO-6973 awarded by The National Cancer Institute, DHHS.
References
I. Thomas ED, Buckner CD, Clift RA et al. (1979) Marrow transplantation
for acute nonlymphoblastic leukemia in first remission. N Engl J
Med 301:597-599
2. Forman SJ, Spruce WE, Farbstein MJ et al. (1983) Bone marrow
ablation followed by allogeneic marrow grafting during first complete
remission of acute nonlymphocytic leukemia. Blood 61: 439-442
3. Powles RL, Morgenstern G, Clink HM et al. (1980) The place of
bone-marrow transplantation in acute myelogenous leukaemia. Lancet
I: 1047-1050
4. Kersey JH, Ramsey NKC, Kim T et al. (1982) Allogeneic bone-marrow
transplantation in acute non-lymphocytic leukemia: A pilot study.
Blood 60:400-403
5. Zwaan FE, Hermans J, for the E.B.M.T. Leukaemia Working Party
(1982) Bone-marrow transplantation for leukaemia. European results
in 264 cases. Exp Hematol 10 (suppl 10):64-69
6. Mannon P, Vemant JP, Roden M et al. (1980) Marrow transplantation
for acute nonlymphoblastic leukemia in first remission. Blut 41:
220-225
7. Gale RP, Kay HEM, Rimm AA et al. (1982) Bone-marrow transplantation
for acute leukaemia in first remission. Lancet 2: 1006 -1009
8. Santos GW, Tutschka PJ, Brookmeyer R et al. (1983) Marrow transplantation
for acute non-lymphocytic leukemia following treatment with busulfan
and cyclophosphamide. N Engl J Med 309: 1347-1353
9. Dinsmore R, Kirkpatrick D, Flumenberg N, Gulati S, Kapoor N,
Brochstein J, Shank B, Reid A, Groshen S, O'Reilly R (1984) Allogeneic
bone-marrow transplantation for patients with acute non-lymphocytic
leukemia. Blood 63:649-656
10. Thomas ED, Sanders JE, Floumoy Net al. (1979) Marrow transplantation
for patients with acute lymphoblastic leukemia in remission. Blood
54:468-476
II. Johnson FL, Thomas ED, Clark BS, Chard RL, Hartmann J R, Storb
R ( 1981) A com parison of marrow transplantation to chemotherapy
for children with acute lymphoblastic leukemia in second or subsequent
remission. N Engl J Med 305: 846-851
12. Scott EP, Forman SJ, Spruce WE et al. (1983) Bone-marrow ablation
followed by allogeneic bone-marrow transplantation for patients
with high-risk acute lymphoblastic leukemia during complete remission.
Transplant Proc 15:1395-1396
13. Kendra JR, Joshi R, Desai Met al. (1983) Bone-marrow transplantation
for acute lymphoblastic leukemia with matched allogeneic donors.
Exp Hematol II (suppl 13): 9 (abstract)
14. Woods WG, Nesbitt NE, Ramsey NKC et al. (1983) Intensive therapy
followed by bonemarrow transplantation tor patients with acute lymphocytic
leukemia in second or subsequent remission: Determination of prognostic
factors (A report from the University of Minnesota Bone Marrow Transplantation
Team). Blood 61: 1182- 1189
15. Dinsmore R, Kirkpatrick D, Flomenberg N et al. ( 1983) Allogeneic
bone-marrow transplantation for patients with acute lymphoblastic
leukemia. Blood 62: 381-388
16. Santos GW (1983) Allogeneic and syngeneic marrow transplantation
for acute lymphocytic leukemia (ALL) in remission. Blood 62:229
17. Thomas E, Storb R (1970) Technique for human marrow grafting.
Blood 36:507-515
|
