|
Evidence that conventional treatment ( chemotherapy and/or immunotherapy) produces a substantial number of cures in acute myeloid leukaemia (AML) is lacking (Powles et al. 1980a). Thomas et al. (1977) showed that there was a proportion of long-term survivors of a group of AML patients treated with high-dose cyclophosphamide, total body irradiation (TBI) and allogeneic bone marrow transplantation (BMT). The purpose of the study reported here is to define the place for BMT in the treatment of AML and involves a comparison of two groups of first remission patients treated either with BMT or chemoimmunotherapy. This study is an extension of the results published earlier this year (Powles et al. 1980c).
Since August 1977 , 33 1st remission AML patients have received a BMT -27 from HLA-identical and MLC-compatible sibling donors, two from identical twin donors and four from related mis-matched donors (three sibling and one paternal, incompatible in MLC). The outcome of the matched allografted patients has been compared with the simultaneous group of 33 patients with AML in first remission, who lacking a suitable donor did not receive a transplant and were maintained on chemotherapy and immunotherapy. Patients details are shown in Table I. Remission was induced by various regimes using cytosine arabinoside, thioguanine and anthracycline-daunorubicin alone or with adriamycin, or rubidazone. Consolidation with the same three agents or with thioguanine and cytosine arabinoside followed. Twenty of the transplanted patients had their remission induction given elsewhere and were referred to our unit when in remission. Those patients who did not receive a transplant were given maintenance chemotherapy consisting of courses of cytosine arabinoside (10 mg/kg as 24 h intravenous infusion) followed by daunorubicin ( 1.5 mg/kg intravenously). given at intervals of 2,4,6,8. 10 and 12 weeks after consolidation for a total period of 42 weeks (Powles et al. 1979). or 3 day courses of cytosine arabinoside ( 1.5 mg/kg sub-cutaneously, 12 hourly) and 6-thioguanine (8O mg orally. 12 hourly) given every 3 weeks for 27 weeks. In addition they received continuous weekly immunotherapy consisting of subcutaneous injections of irradiated myeloblasts and intradermal BCG . Table I. Details of patients studied
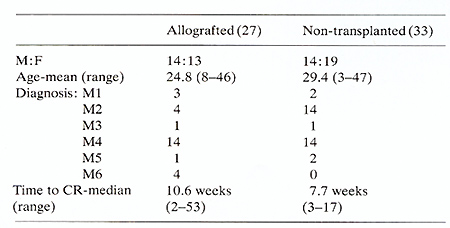
Actuarial analysis of complete remission duration is shown in Figure
1. Of the 33 patients treated with chemo-immunotherapy 20 have relapsed
with a median remission duration of 12 months compared with four
of the matched allografted patients. This difference is highly significant
(P<0.001) using the log-rank test. Twelve of the 33 chemo-immunotherapy
patients have died (11 of relapse and one of infection while in
remission) with a median survival of 21 months from date of complete
remission. There have been five deaths in the 27 matched allografted
patients, one of relapse, four of GVHD with or without pneumonitis,
( two of these patients received methotrexate prophylaxis and one
only a short course of CSA). Actuarial analysis of survival from
complete remission of these two groups (Fig. 2) shows that while
there is not at present a statistically significant difference between
them, at no time do the transplanted patients fare worse and they
have a 75% 3 year actuarial survival compared with 45% for the chemoimmunotherapy
patients. Survival from graft date is shown in Figure 3. Nineteen
(70% ) matched allografted patients remain alive in continuous remission
compared with 12 (33%) of chemoimmunotherapy patients (P= <0.01
). The syngeneic and mis-matched allografts received the same conditioning
regimen and supportive care. The mis-matched allografts all received
prolonged CSA as prophylaxis against GVHD. Their outcome is shown
in Table 2. 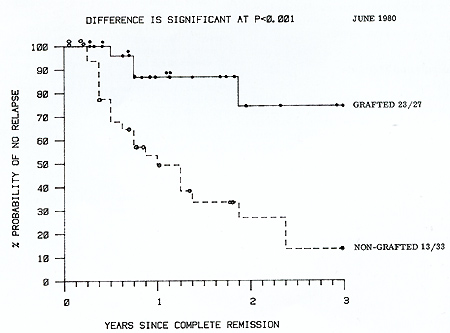 Fig. I. Actuarial life table analysis of complete remission duration
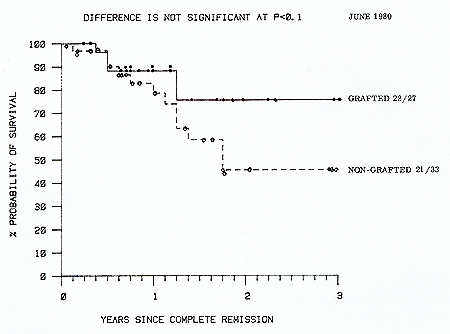 Fig. 2. Actuarial life table analysis of survival from
complete remission date 
Table 2. Outcome of patients transplanted
for AML in first remission 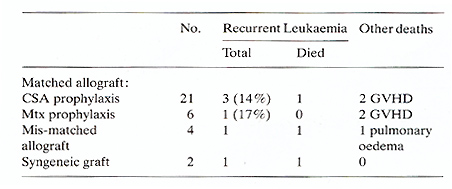
Powles RL, Selby PJ, Palu G, ( 1979) The nature of remission in
aeute myeloblastic leukaemia. Lancet 11:674-76 |