In: Zander et al. (eds) Gene Technology, Stem Cell and Leukemia Research,Nato Asi Series H: Cell Biology, Vol 94,Springer-Verlag, Berlin Heidelberg New York London |
|
for the Polish Acute Leukemia Group* Intensive induction chemotherapy regimens has led to considerable improvement in the prognosis of adult acute lymphoblastic leukemia ( ALL ), but the results are still unsatisfactory and only about 35% of patients can be cured with current available therapy (7,10,11,14, ) .In conclusions from the studies of large series of adult ALL the complete remission (CR) rate ranges from 70 to 85% ( 2,6,7, 9,10, 11, 14,16,23,27,30 ). A further intensification of chemotherapy will increase CR rates but it is limited by hematopoietic toxicity and therefore the use of recombinant human growth factors in addition to induction chemotherapy has been attempted ( 13,18,19,22,23,25,29 ). The studies on bone marrow cells kinetics during GM-CSF treatment revealed, that 48 to 96 hours after GM-CSF discontinuation the proliferative activity of normal stem cells and progenitors decreases to levels lower than that observed prior to GM-CSF administration ( 1,5, 12,28 ), so this represents a period of partial refractoriness of these cells to the cell cycle dependent cytostatics. Based on the above data as well as on pharmacokinetics of anthracyclines and after successful completing of the pilot study ( 12 ), an original cell kinetics based protocol using rhGM-CSF during induction and consolidation treatment of adult ALL has been designed and evaluated in a multicenter, randomized, prospective study. The aims of this study were as follows: 1) to evaluate the efficacy and tolerability of recombinant human GM-CSF in adult ALL patients, 2) to prove the concept that sequential administration of cell cycle specyfic cytostatics and GM-CSF during induction can enhance the myeloprotection by modulation of normal stem cell kinetics, 3) to evaluate the efficacy of GM-CSF for prevention of neutropenia after intensive consolidation therapy. Material and methods. The multicenter, prospective, randomized trial, using GM-CSF in adult ALL patients has been carried out in 8 departments of haematology. Recombinant human GM-CSF (E.coli derived, Leucomax ) had been supplied by Sandoz/Schering Plough. Fifty two consecutive, previously untreated ALL adult patients were enrolled to the study ( F=22,M=30,median age 29 (range16-59), immunological phenotypes: common=35, early pre 8=7, pre TIT type=1 0, median WBC count=9,4 G\L, median Hb=9,35 g/l, median platelets count=59 G\L, hepatomegaly presented 63% of patients, splenomegaly-65%, Iymphomegaly- 71% of patients) Patients below 16 years and over 60 years old, FAB L3,ALL with "myeloid" suface antigens, historyof anaphylaxis to human proteins, with severe heart, liver,lung 0r kidney impairment, were the exclusion criteria. After informed consent had been obtained, patients were stratified according to risk groups the high risk one (age>35, WBC>30 G\L, "common" negative, phenotype Ph+) and the standard risk one (none of the above criteria). Within each group patients were randomized to receive either chemotherapy plus GM-CSF (GM arm) or chemotherapy alone (control arm). The patient's characteristics in each group is presented in the table 1. tab. 1 Material 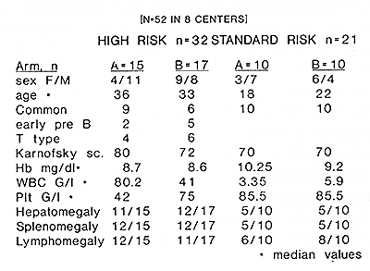 The patients treated according to the schedule presented in fig. 1. 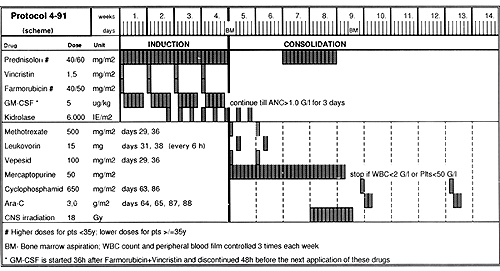
Recombinat human GM-CSF ( Leukomax ) , was subcutaneously, in daily dose of 5 ug/kg body weight , during induction, in five day cycles, each one was startet 36 hours after Farmorubicin and Vincristin injection and discontinued 48 hours before the next injection of cytostatics. After completion of the induction, as well as during consolidation with Cyclophosphamide and HO ARA-C, GM-CSF was continued until absolute neutrophil count (ANC) in peripheral blood reached 1 G\l during three consecutive days. The peripheral blood morphology with differential count, reticulocytes and platelets were monitored three times a week. In addition, the monitoring of peripheral blood CO34,CO38 positive cells was performed in 13 patients; 8 receiving GM-CSF and 5 treated in the control arm (each time on the last day of GM-CSF administration -days 3,20,27, and next after 48 hours, just before cytosta-tics dministration -days 1,8,15,22. lmunophenothyping of leukemic blasts at the start of induction and next peripheral blood active stem cells measurements during induction performed using flow cytometry immunoenzymatic APAAP staining. The statistal analysis of survival was performed using original database programme leukos 7 and Biomedical Package Software BMDP.
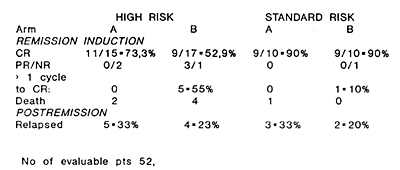
The overall CR rate equalled 73%, with 90% CR in the standard risk group and 62,5% in the high risk one. A higher CR rate of 80% has been obtained in patiente treated with GM-CSF (GM arm) as compared to the control group -66%. All patients in the "GM arm" achieved CR after 1 induction cycle, whereas 33% of patients in the control arm received more than 1 cycle of induction. The time to reach CR in the "GM arm" was significantly shorter (median 29 days) than in the control one (median 41 days) p<0.05. The particular results obtained in each group of patients are schown in table 2. tab. 2 Results of induction treatment
 Analysis of bone marrow regeneration times revealed, that in the GM arm both the cytoreduction and the subsequent regeneration of particular haematopoetic lines occured significantly earlier than in the control arm (median of days to nadir granulocyte counts equaled 9 versus 15 days, p<0.05 and to nadir platelets 6.5 versus 12,5 days respectively, p<0.05 ). Times to granulocyte recovery and surprisingly also to platelets recovery were also significantly shorter in the "GM arm" than in the control one; median days to ANC >0.5 G\L 11 versus 28, to > 1 G\L 21,5 versus 33,5, to > 1,5 G\L 24 versus 34 days, p<0.05 , median of days to reach platelet count >50 G\L 8.5 versus 12 days, > 100 G/I 26,5 versus 33,5 days (p<0.05. The time to clearance of blasts from peripheral blood was also shorter in the GM arm (median 7 days) as compared to the control group (10 days). 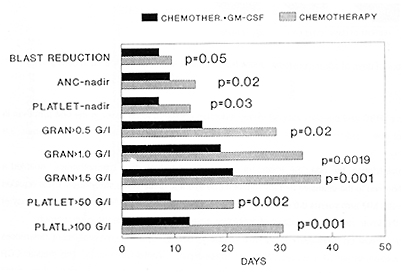
The use of GM-CSF shortened significantly the neutropenic period
during induction, the mean of days with ANC < 0.5 G\L in GM arm
equaled 10,6 as compared to 21,4 in the control arm (p<0.05) and
only 59% of GM-CSF treated patients displayed agranulocytosis, whereas
85% in the control group. The insidence and severity of infections
during induction were reduced in the GM-CSF treated group (only
9% of patients in GM arm developed infection of WHO grade 4, compared
to 23% in the control arm). As a consequence also the antibiotic
treatment was significantly reduced in the GM arm (p<0.05) fig 3.
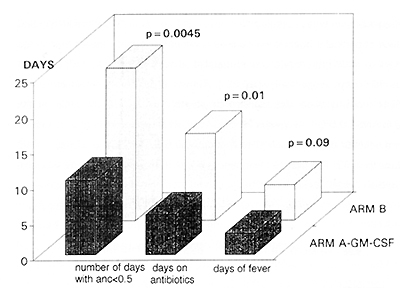
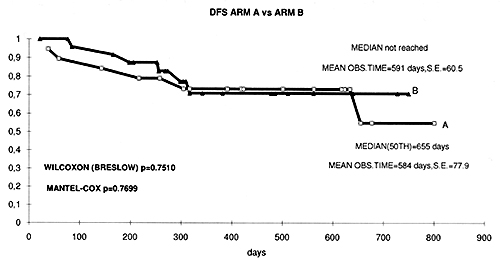 fig. 4 Kaplan Meyer curves of disease free survival in both groups of patients
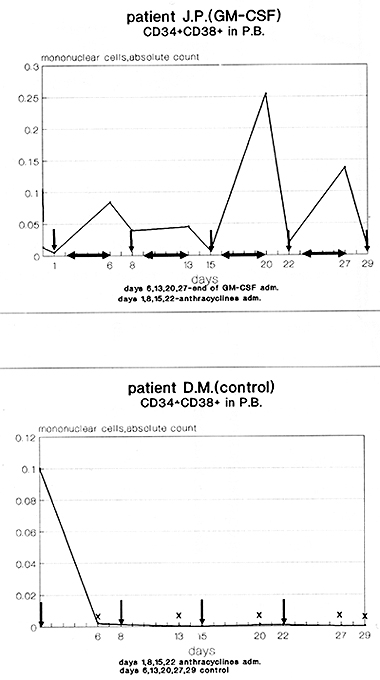 fig. 5 The kinetics of peripheral blood CD34+CD38+cells during GM-CSF and chemotherapy treatment compared to chemotherapy alone
Discussion The current results of the study presented here support the idea of the beneficial use of growth factors in combination with chemotherapy in acute lymphoblastic leukemia. The results of multiple studies reflect the impact of the use intensive chemotherapy regimens in induction of ALL (2,6,7,9,10,11,13,14,16,18,22,23,25,27,30) to achieve rapid cytoreduction, but it is important balance the antileukemic effect with the minimal myelotoxicity (14). In the German Multicentre Adult ALL Trial the granulocytopenia has appeared the major dose limiting toxicity, leading to delay or discontinuation of therapy and most investigators have demonstrated that an induction failure is equally due to the toxicity and to the refractoriness t0 chemotherapy (14). The use of recombinant growth factos such G-CSF and GM-CSF in the management of lymphoid malignancies have demonstrated their effectiveness in supportion of myelopoiesis, a good tolerability (4,8,11,13,18,24,25) with no evidence of malignant lymphoid clones stimulation (1,3,15,17,20,26). In addition to supportive function of growth factors in cytopenia, new perspectives have been offered based on prewious data suggesting that 48-96 hour period after discontinuation of GM-CSF administration represents a period of relative refractoriness of normal progenitors to cell cycle specyfic chemotherapy (1,3,5,12,26,28). Based on these findings we developed a novel remission induction protocol, and the data presented here confirm its efficacy. Our flow cytometric findings of kinetic charnges of peripheral blood CD34 CD38 posivite cells during and after GM-CSF treatment appear to confirm that a period of relative refractoriness of normal stem and progenitor cells may be obtained using properly sequenced administration of rhGM-CSF and cytostatic drugs. Rapid granulopoietic reconstitution, as well as a rather unexpected shorter platelets recovery time, achieved in GM-CSF group should be considered as an another evidence of cytoprotection on multilineage stem cell level. Complete remission has been achiewed significantly more rapidly in patients receiving GM-CSF in addition to chemotherapy and this finding is of great importance. At first it confirs the value of the above discussed policy and secondly it has a prognostic value predicting for a longer remission duration. Data from large series of ALL patients demontrated that a short induction treatment duration , is an one of the most important parameters predicting long term disease free survival ( 9, 10, 11 ). Further investigations are needed to explain the accelerated reduotion of leukemic cells during GMCSF administration. A priming effect of secondary secreted cytokines as well as activation of immunological mechanisms of tumor control should be taken into consideration. In summary, the protocol employing GM-CSF has following advantages in comparison to chemotherapy alone: shorter time to CR, (p<0.05), CR in all cases obtained after 1 chemotherapy cycle, accelerated blasts cytoreduction (p=0.05), accelerated hematopoietic reconstitution ( p<0.05 ), better outcome of treatment with lower incidence and severity of infections. The use GM-CSF during high dose consolidation treatment permits close adherence to planned chemotherapy without delay. Our clinical data supported by flow cytometric findings confirm the idea of a cell kinetics based protection of stem cells in ALL, using properly sequenced chemotherapy and GM-CSF . The study protocol received the Local Ethics Commitee agreement.
Aglietta M. Piacibello W.et al.Kinetics of Human Hematopoietic
Cells After in Vivo Administration of Granulocyte Macrophage Colony
Stimulating Factor J.Clin.lnvest.1989.83. |